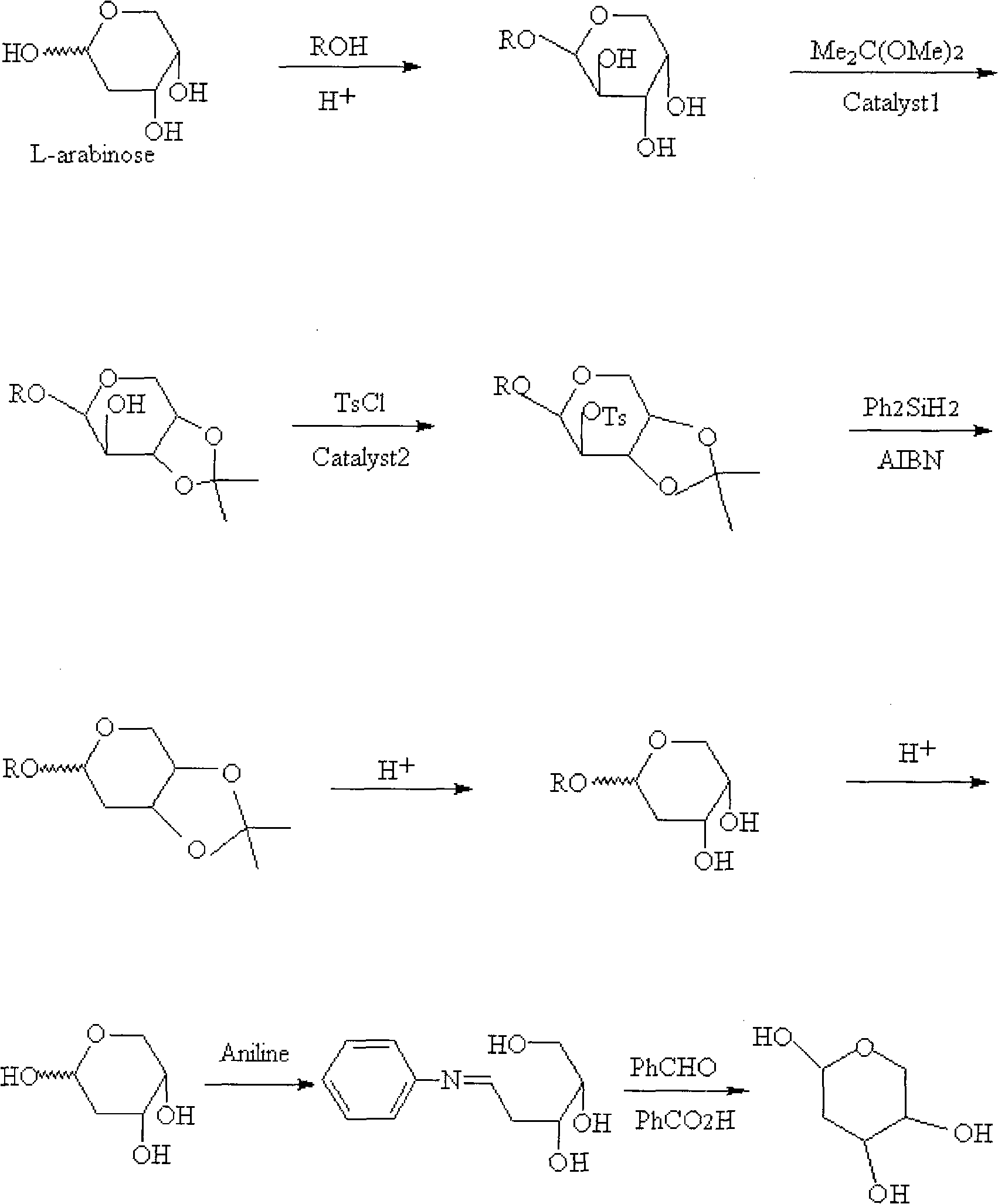
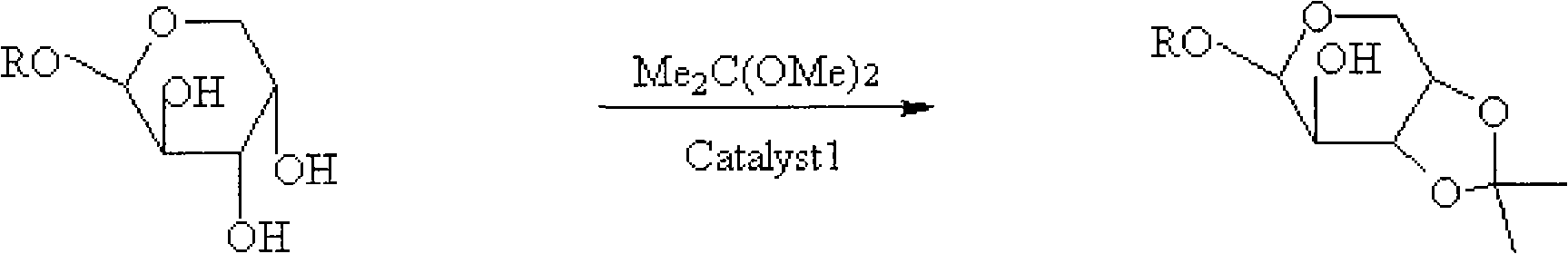Method for preparing 2-deoxidation-L-ribose
A technology of ribose and arabinoside, which is applied in the field of preparation of 2-deoxy-L-ribose, can solve difficult problems such as separation and purification, and achieve the effect of easy reaction and purification conditions, cheap price, and less "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method process of 2-deoxy-L-ribose of the present invention is as follows:
[0024] 1) Preparation of 1-O-methyl-L-arabinoside
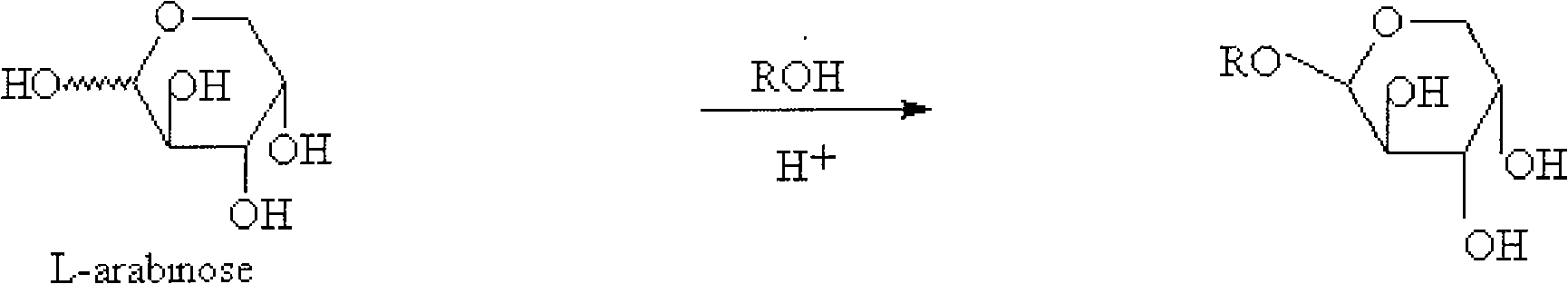
[0025] At room temperature, add L-arabinose to 4 times the mass of saturated hydrogen chloride methanol solution. The mixture was heated to 63°C-65°C under reflux for 2-4 hours, and cooled to room temperature. The solution was concentrated to 1 / 2 the original volume to give a suspension. Filter out the solid precipitate, continue to concentrate the liquid to 1 / 4 of the original volume, filter out the solid precipitate, wash all the precipitate with 0.5 times the mass of methanol, and dry to obtain 1-O-methyl-L-arabinoside, the yield 89.7%.
[0026]
[0027] R: -CH 3
[0028] 2) Preparation of 1-O-methyl-3,4-O-isopropylidene-L-arabinoside
[0029] Dissolve the 1-O-methyl-L-arabinoside obtained in the previous step in 5 times the mass of dimethylformamide (DMF), add 2 times the mass of the reaction reagent dimethoxypropa...
Embodiment 2
[0052] The preparation method process of 2-deoxy-L-ribose of the present invention is as follows:
[0053] 1) Preparation of 1-O-ethyl-L-arabinoside
[0054] At room temperature, add L-arabinose to 4 times the mass of saturated hydrogen chloride ethanol solution. The mixture was heated to 63°C-65°C under reflux for 2-4 hours, and cooled to room temperature. The solution was concentrated to 1 / 2 the original volume to give a suspension. Filter out the solid precipitate, continue to concentrate the liquid to 1 / 4 of the original volume, filter out the solid precipitate, wash all the precipitate with 0.5 times the mass of ethanol, and dry to obtain 1-O-ethyl-L-arabinoside, the yield 90.1%.
[0055]
[0056] R: -CH 2 CH 3
[0057] 2) Preparation of 1-O-ethyl-3,4-O-isopropylidene-L-arabinoside
[0058] Dissolve the 1-O-ethyl-L-arabinoside obtained in the previous step in 5 times the mass of dimethylformamide (DMF), add 2 times the mass of the reaction reagent dimethoxypropa...
Embodiment 3
[0081] The preparation method process of 2-deoxy-L-ribose of the present invention is as follows:
[0082] 1) Preparation of 1-O-n-propyl-L-arabinoside
[0083] At room temperature, add L-arabinose to 4 times the mass of saturated hydrogen chloride in n-propanol. The mixture was heated to 63°C-65°C under reflux for 2-4 hours, and cooled to room temperature. The solution was concentrated to 1 / 2 the original volume to give a suspension. Filter out the solid precipitate, continue to concentrate the liquid to 1 / 4 of the original volume, filter out the solid precipitate, wash all the precipitate with 0.5 times the mass of n-propanol, and dry to obtain 1-O-n-propyl-L-arabinoside , yield 89.5%.
[0084]
[0085] R: -CH 2 CH 2 CH 3
[0086] 2) Preparation of 1-O-n-propyl-3,4-O-isopropylidene-L-arabinoside
[0087] Dissolve the 1-O-n-propyl-L-arabinoside obtained in the previous step in 5 times the mass of dimethylformamide (DMF), add 2 times the mass of the reaction reagent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com