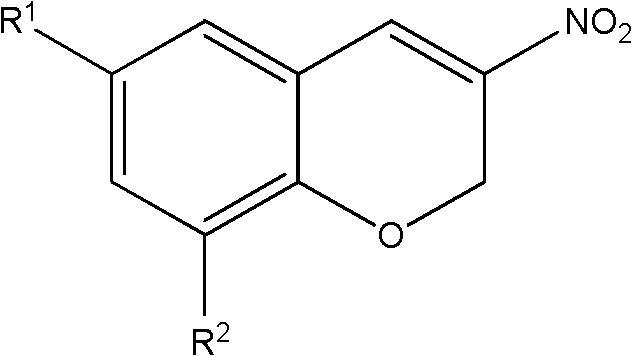
3-nitro-2h-chromene compounds with antibacterial activity and preparation method and application thereof
The technology of a compound and a nitro group is applied in the field of 3-nitro-2H-chromene compound and its preparation, which can solve the problems of prolonging the treatment time of patients, increasing the mortality rate and the like, and achieving the effect of simple reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
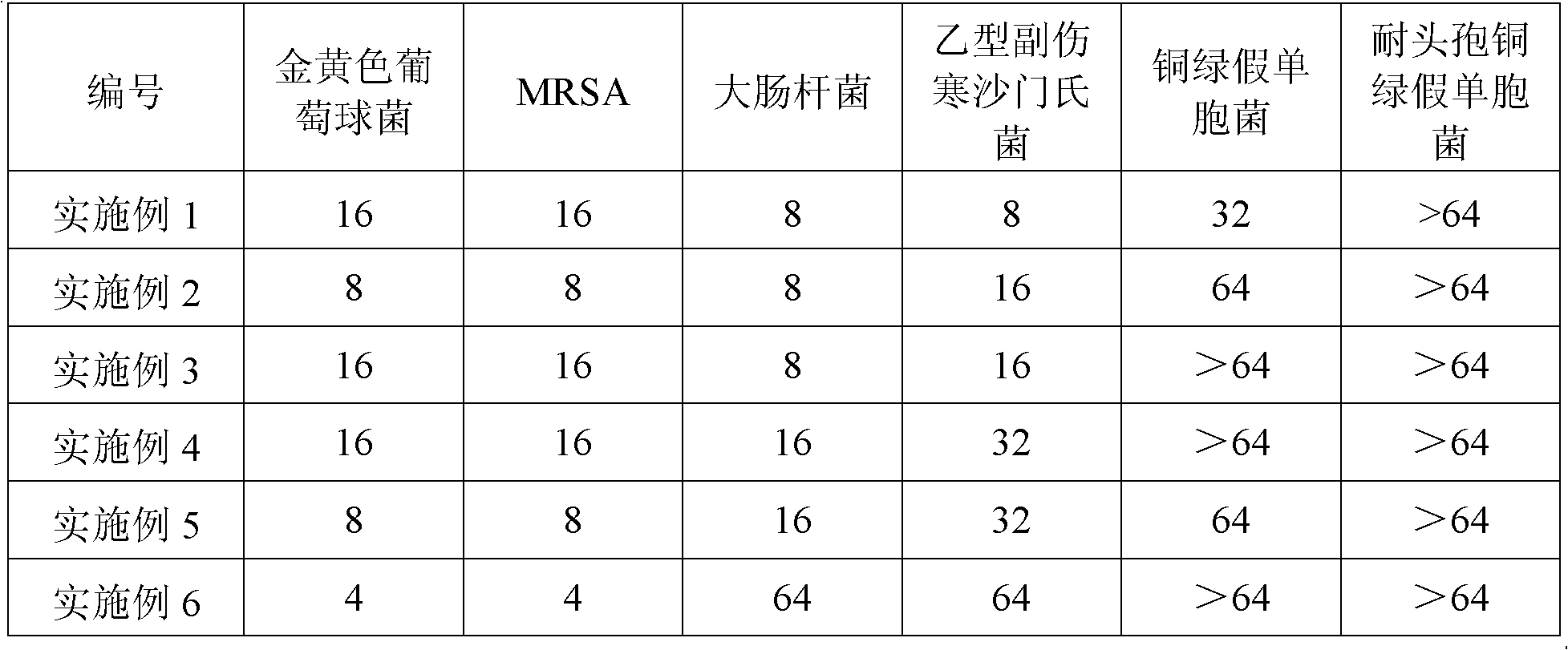
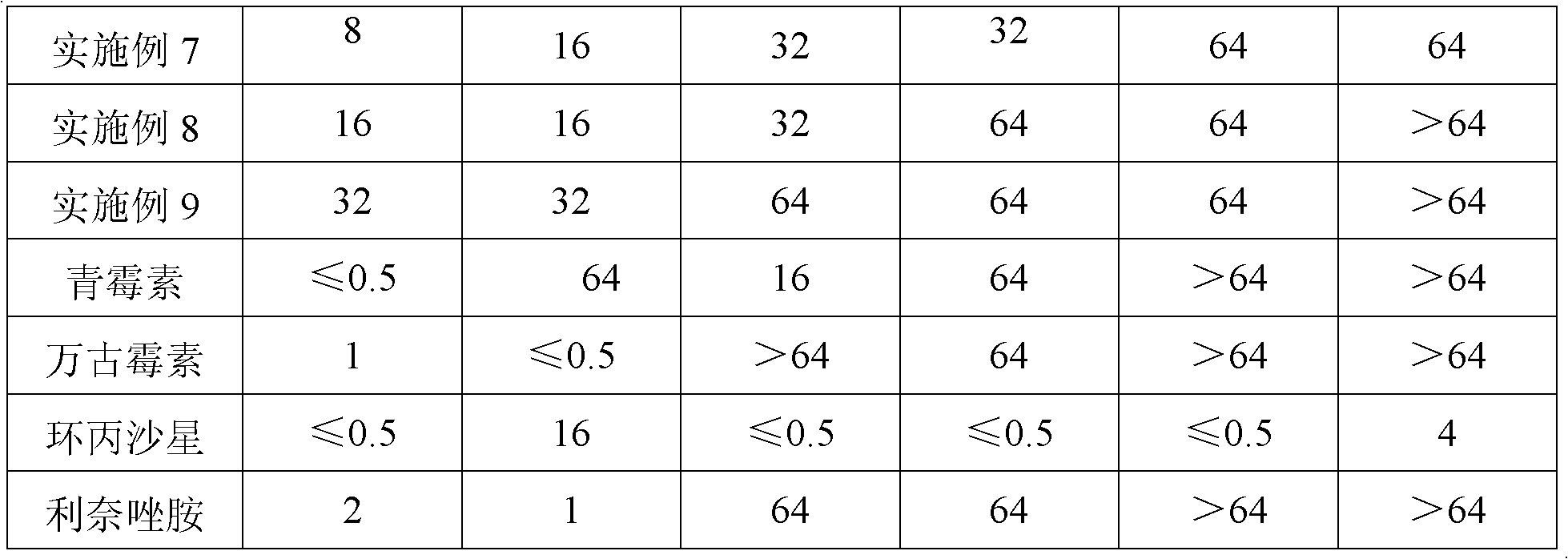
Examples
Embodiment 1
[0019] Example 1: 3-nitro-2H-chromene
[0020] In a 250mL three-neck round bottom flask, add salicylaldehyde (0.488g, 4mmol), dibutylamine (0.260g, 2mmol), phthalic anhydride (1.184g, 8mmol) and toluene (25ml), and then load Dean-Stark water splitter. 2-Nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours) with an automatic syringe pump, and the reaction mixture was stirred and refluxed for 24 hours. Then 2-nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours), and the reaction mixture was refluxed for 24 hours. After removing the solvent under reduced pressure, the crude product was purified by silica gel column chromatography (petroleum ether / dichloromethane gradient elution) to obtain 0.324 g of a yellow solid product, namely 3-nitro-2H-chromene, with a yield of 45.8% and a melting point of 77.0 -78.0°C. The characterization parameters of 3-nitro-2H-chromene are as follows:
[0021] 1 H NMR (400MHz, CDCl 3 )δ: 7.79(s, 1H), 7.37-7.32(m, 1H), 7.26(dd, J=7.6, 1...
Embodiment 2
[0023] Example 2: 3-nitro-6-chloro-2H-chromene
[0024] In a 250mL three-neck round bottom flask, add 5-chloro-2-hydroxybenzaldehyde (0.626g, 4mmol), n-Bu 2 NH (0.260 g, 2 mmol), phthalic anhydride (1.184 g, 8 mmol), and toluene (25 ml) were fitted with a Dean-Stark trap. 2-Nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours) with an automatic syringe pump, and the reaction mixture was stirred and refluxed for 24 hours. Then 2-nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours), and the reaction mixture was refluxed for another 24 hours. After removing the solvent under reduced pressure, the crude product was purified by silica gel column chromatography (petroleum ether / dichloromethane gradient elution) to obtain 0.372 g of 3-nitro-6-chloro-2H-chromene as a yellow solid, with a yield of 44.0% , melting point: 109.5-110.4°C.
[0025] 1 H NMR (400MHz, CDCl 3 )δ: 7.71(s, 1H), 7.29(dd, J=8.7, 2.5Hz, 1H), 7.24(d, J=2.5Hz, 1H), 6.87(d, J=8.7Hz, 1H), 5.26( d, J=1.1...
Embodiment 3
[0027] Example 3: 3-nitro-6-fluoro-2H-chromene
[0028] In a 250mL three-neck round bottom flask, add 5-fluoro-2-hydroxybenzaldehyde (0.560g, 4mmol), dibutylamine (0.260g, 2mmol), phthalic anhydride (1.184g, 8mmol) and toluene (25ml), and then install the Dean-Stark water separator. 2-Nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours) with an automatic syringe pump, and the reaction mixture was stirred and refluxed for 24 hours. Then 2-nitroethanol (0.364 g, 4 mmol) was added slowly (12 hours), and the reaction mixture was refluxed for 24 hours. After removing the solvent under reduced pressure, the crude product was purified by silica gel column chromatography (petroleum ether / dichloromethane gradient elution) to obtain 0.367 g of orange solid, namely 3-nitro-6-fluoro-2H-chromene, yield 47.1 %, 85.5-86.5°C.
[0029] 1 H NMR (400MHz, CDCl 3 )δ: 7.72(s, 1H), 7.07-7.02(m, 1H), 6.98(dd, J=7.8, 3.0Hz, 1H), 6.89(dd, J=8.9, 4.4Hz, 1H), 5.23(d , J=1.0Hz, 2H); 13 C NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com