Method for improving expression level of Pichia pastoris recombinant protein
A recombinant protein, Pichia pastoris technology, applied in the field of bioengineering, can solve the problems affecting the expression of exogenous proteins, yeast cell free radical accumulation, yeast death and other problems, and achieve the effect of overcoming free radical accumulation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
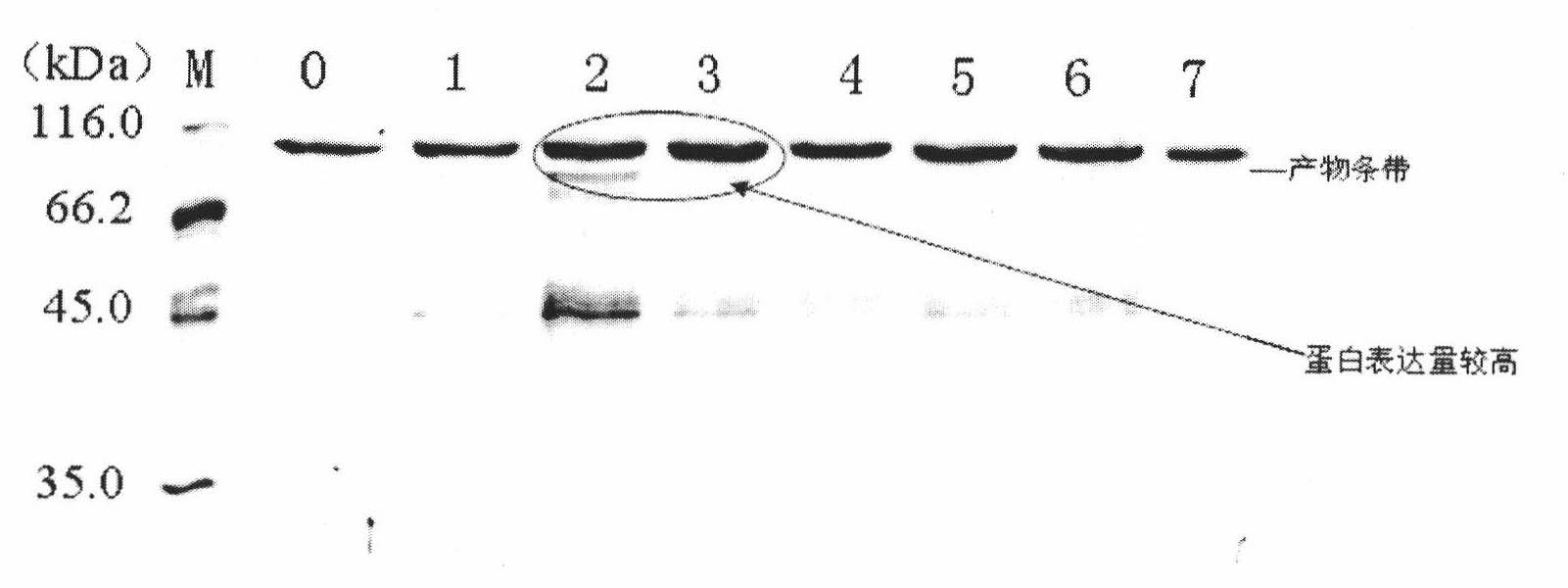
[0014] The engineered bacteria GS115 / pPIC9K-IL2-HSA expressing interleukin-2-human serum albumin fusion protein was constructed and preserved in our laboratory, and the methanol utilization phenotype of the recombinant Pichia pastoris was mut + .
[0015] (1) Pick a single colony from the plate where the high-expression strain is preserved, place it in a 50 mL shake flask containing 10 mL of LYPD medium, and culture it at 30° C. and 200 rpm for 24 hours. (2) The bacteria cultured in YPD medium for 24 h were inoculated into a 1000 mL shake flask containing 200 mL of BMGY medium at a ratio of 1:100, and cultured at 30° C. and 200 rpm for 48 h. During this period, 1 mL of bacterial suspension was taken every 4 hours, and the bacterial concentration was detected by ultraviolet spectrophotometer (bacterial concentration is expressed as A 600 is the counting unit), and draw the growth curve.
[0016] (3) Repeat steps (1) and (2). When the bacteria grow to 36 hours, put the bacteri...
Embodiment 2
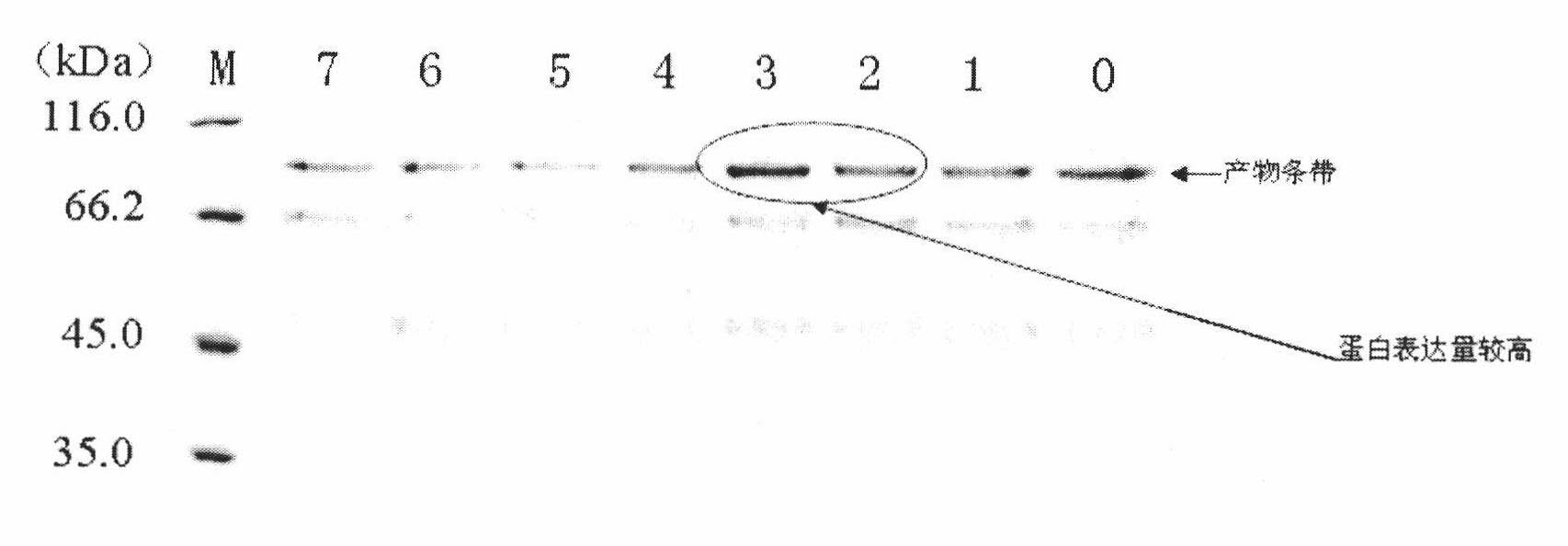
[0018] The engineered bacteria GS115 / pPIC9K-IFNβ-HSA expressing β-interferon-human serum albumin fusion protein was constructed and preserved in our laboratory, and the methanol utilization phenotype of the recombinant Pichia pastoris was mut + .
[0019] (1) Pick a single colony from the plate where the high-expression strain is preserved, place it in a 50 mL shake flask containing 10 mL of LYPD medium, and culture it at 30° C. and 200 rpm for 24 hours. (2) The bacteria cultured in YPD medium for 24 h were inoculated into a 1000 mL shake flask containing 200 mL of BMGY medium at a ratio of 1:100, and cultured at 30° C. and 200 rpm for 48 h. During this period, 1 mL of bacterial suspension was taken every 4 hours, and the bacterial concentration was detected by ultraviolet spectrophotometer (bacterial concentration is expressed as A 600 is the counting unit), and draw the growth curve.
[0020] (3) Repeat steps (1) and (2). When the bacteria grow to 36 hours, put the bacteri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com