Preparation method of atorvastatin calcium isomer mixture and its intermediate
A technology of isomer mixtures and compounds, applied in the field of chemical synthesis, can solve problems such as diastereoisomer synthesis and analysis not involved, quality control methods are complicated, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
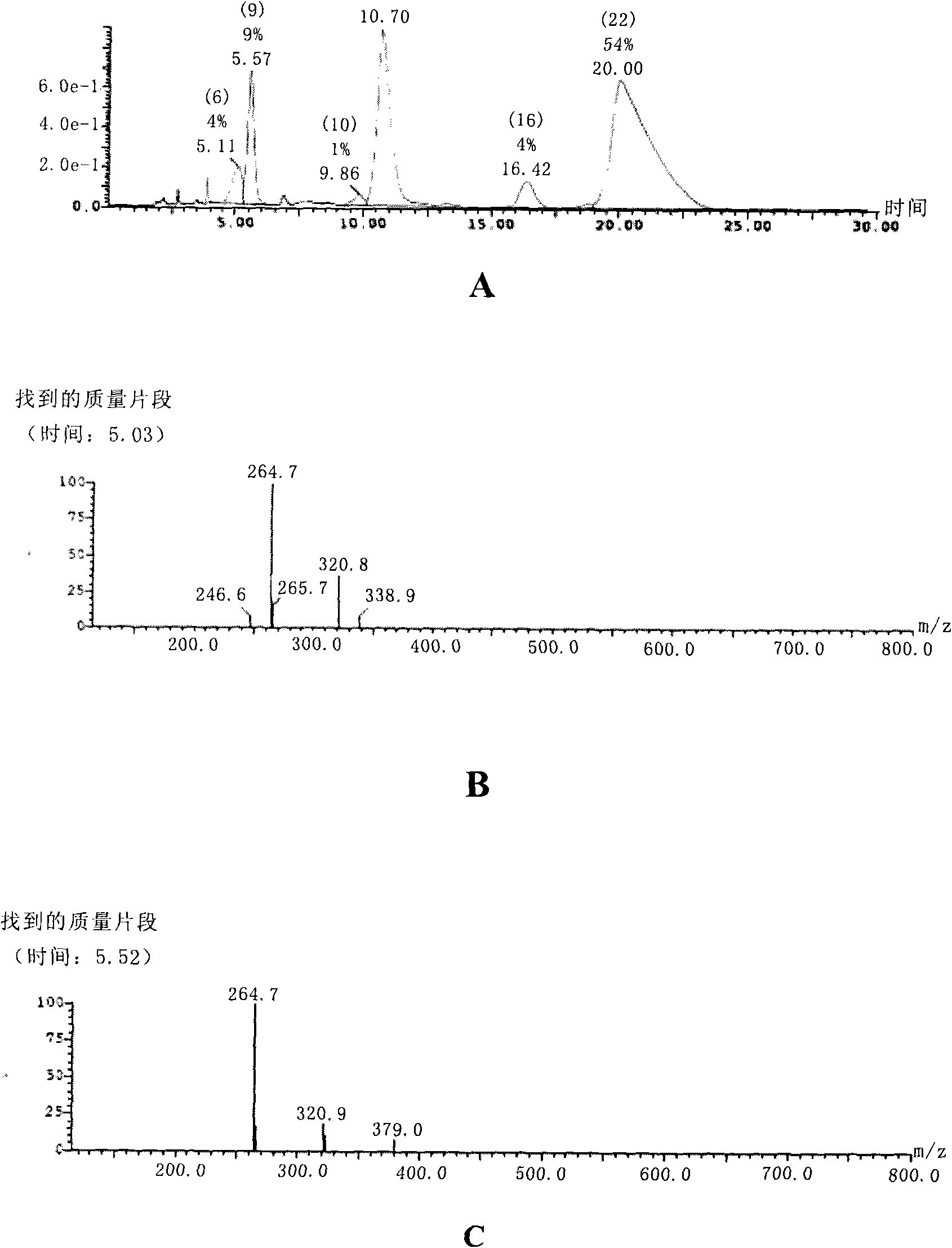
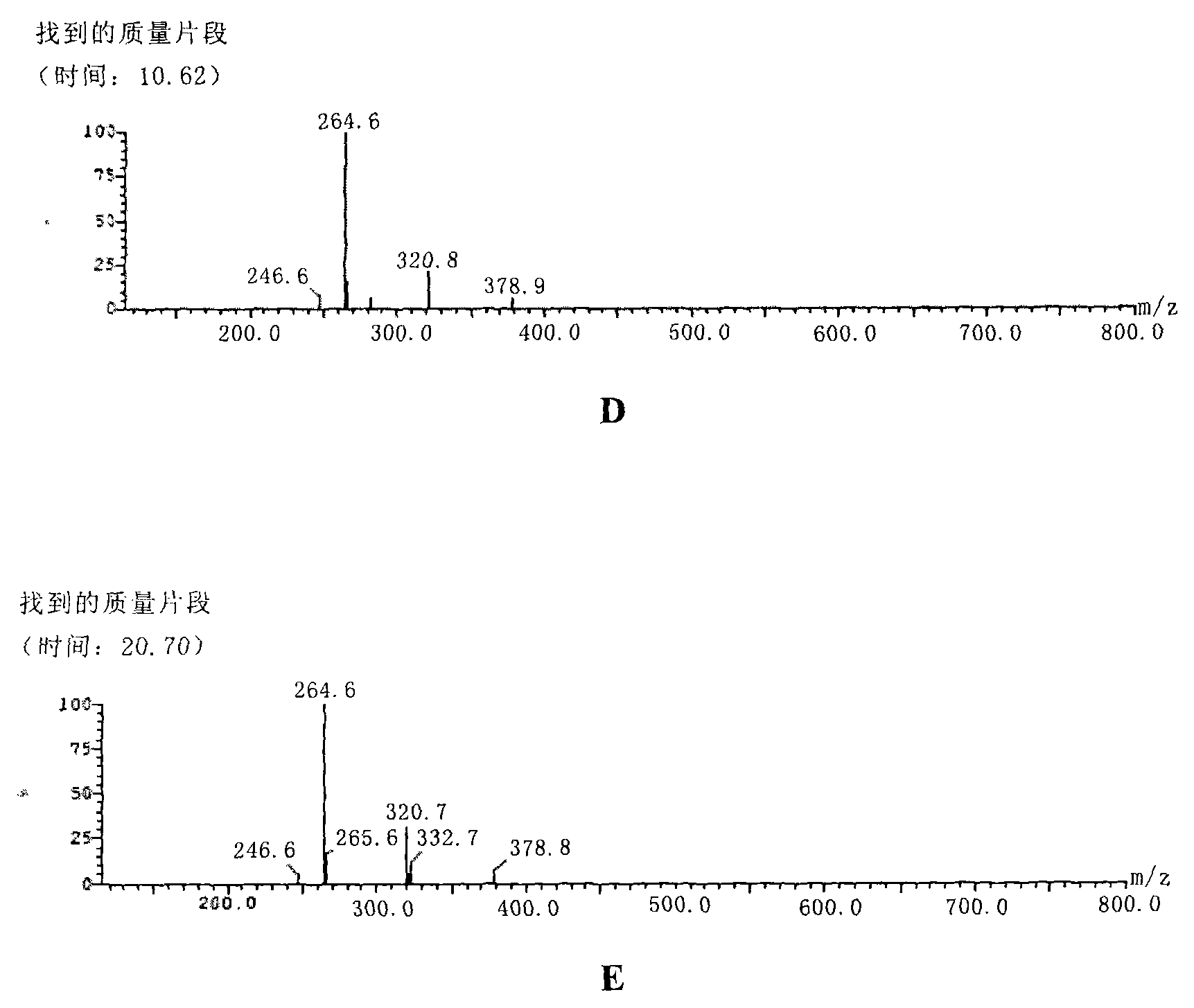
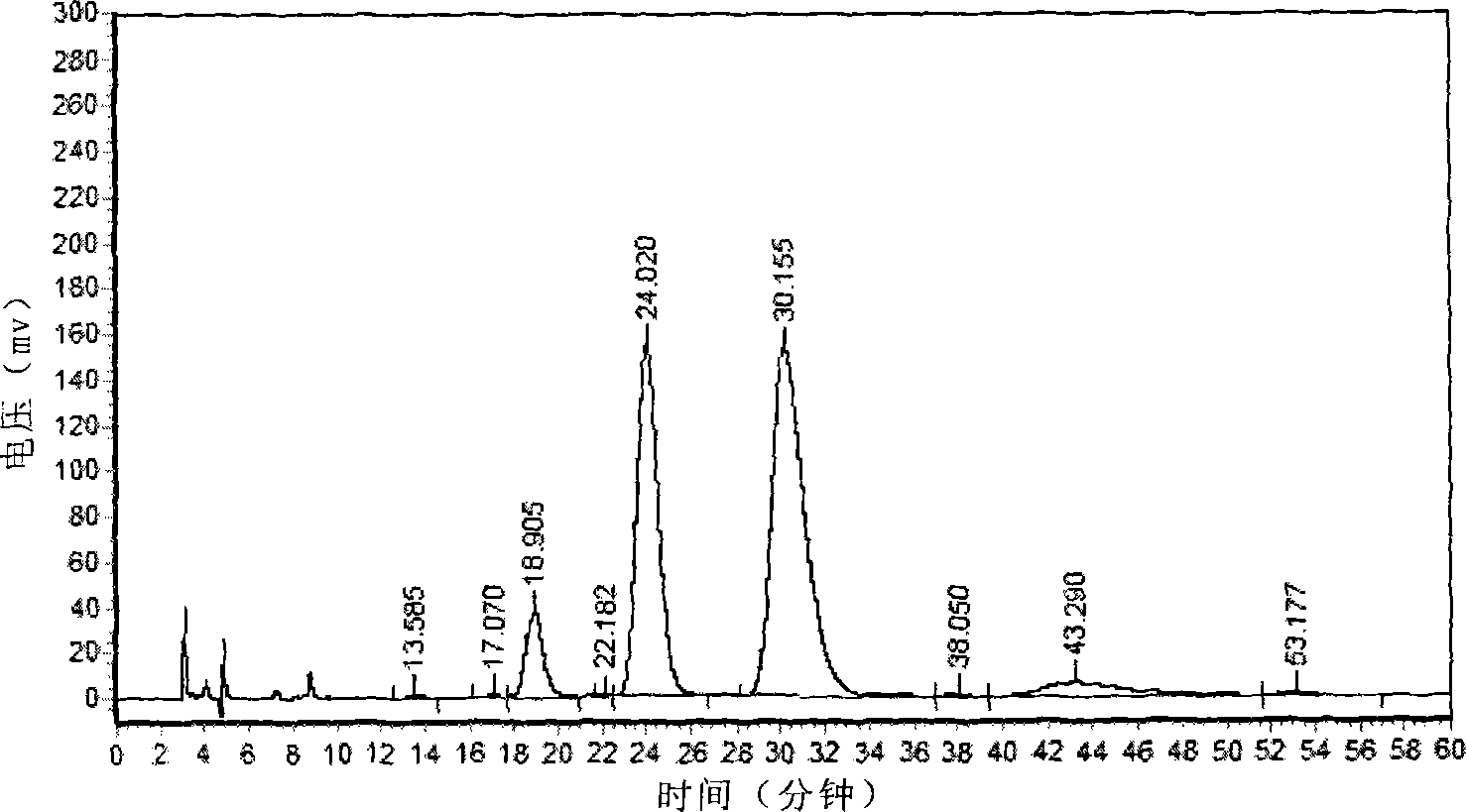
Image
Examples
preparation example Construction
[0074] The present invention provides a method for preparing a mixture of isomers as shown in formula 11A, the method comprising the steps:
[0075] (a) Mixing the compound shown in formula 2 and compound RCOX to obtain the compound shown in formula 7A;
[0076] (b) Ring-opening of the compound represented by formula 7A in the presence of an acid to obtain the compound represented by formula 8A;
[0077] (c) mixing the compound shown in formula 8A with an oxidizing agent to obtain an isomer mixture shown in formula 9A;
[0078] (d) reducing the compound shown in formula 9A to obtain an isomer mixture shown in formula 10A; and
[0079] (e) Mixing the isomer mixture of the compound shown in formula 10A, the catalyst, acetone and 2,2-dimethoxypropane to obtain the isomer mixture shown in formula 11A;
[0080] Wherein R is an ultraviolet emitting group, aryl; aryloxy;
[0081] X is fluorine, chlorine, bromine, or iodine.
[0082]
[0083] In a preferred embodiment of the present invention, the...
Embodiment 1
[0152] Compound 2 is reacted with benzoyl chloride to obtain compound 3. The molar ratio of compound 2 to benzoyl chloride is 1:1. Dissolve 2 (10g, 0.037mol) in dichloromethane and add dropwise benzoyl chloride (5.18g, 0.037mol). ), control the temperature not to exceed 40°C, and stir at 30°C for 2h after the addition is complete. Water was added for washing, liquid separation, the organic phase was dried with anhydrous sodium sulfate and concentrated to obtain 10.1 g of the crude product of compound 3 with a yield of 72%.
Embodiment 2
[0154] Dissolve compound 3 (10.1g, 0.027mol) in methanol, add dropwise 10% hydrochloric acid, the molar ratio of 10% hydrochloric acid to compound 3 is 2:1, stir after the dropwise addition for 2-3h, add saturated sodium bicarbonate solution to adjust After the pH reached 6-7, it was concentrated, and dichloromethane was added for liquid separation. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 8.5 g of crude isomer mixture 4 with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com