N-substituted alpha-amino acid derivatives and preparation method and application thereof
A technology of amino acids and derivatives, which is applied in the directions of drug combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
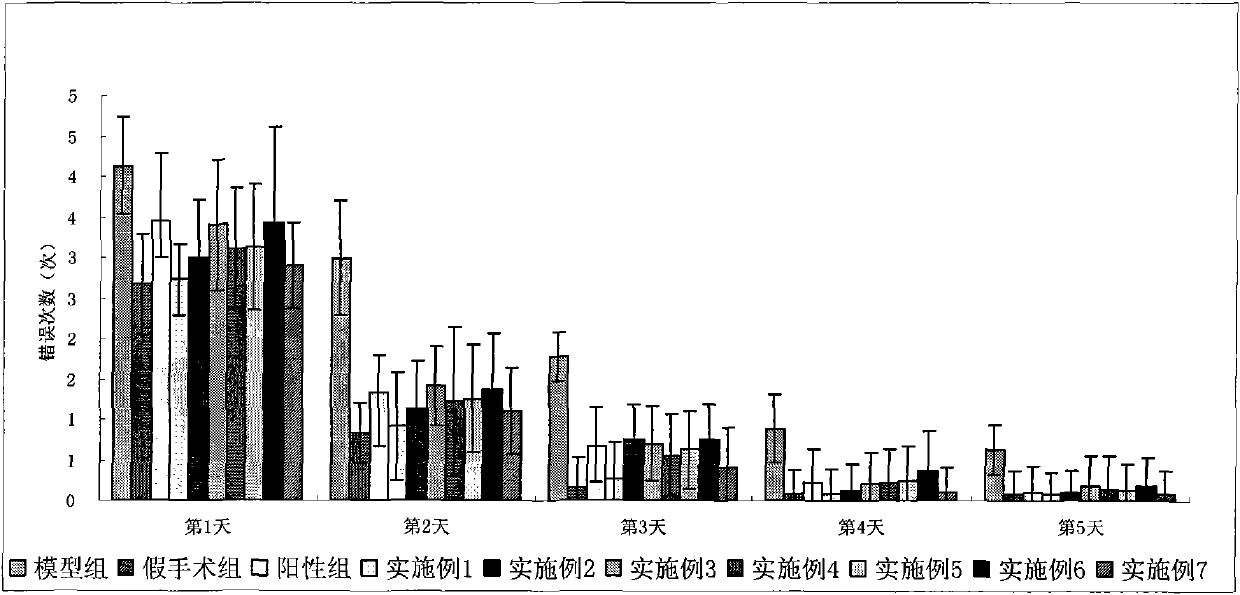
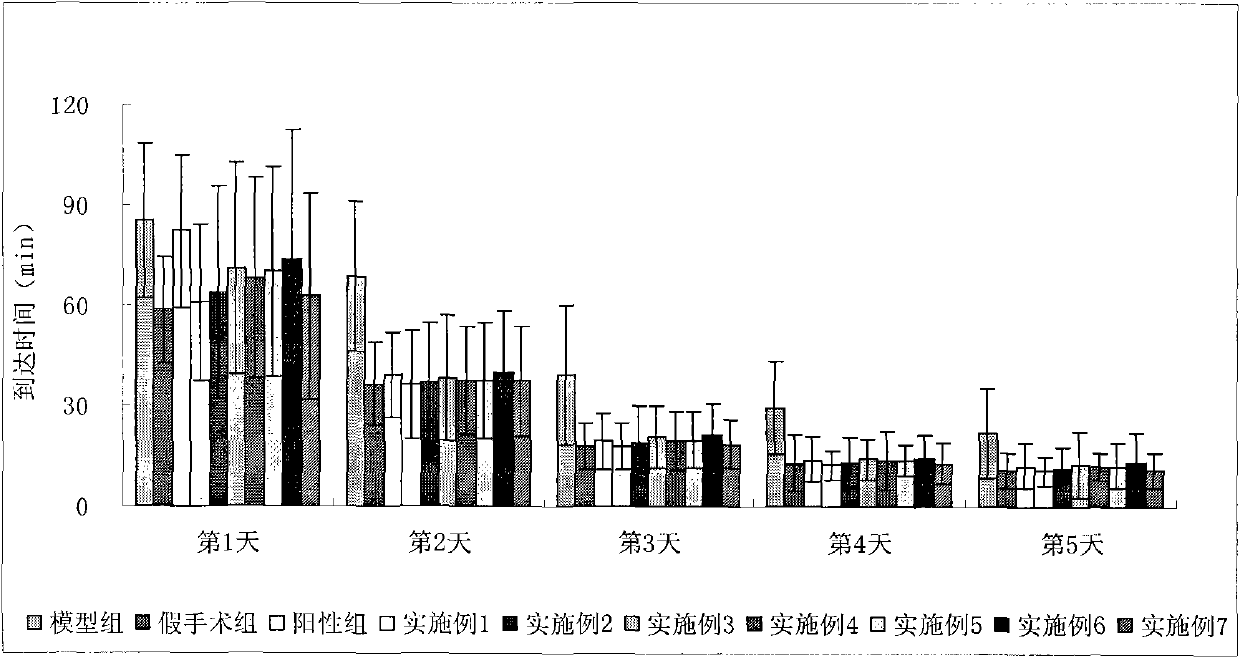
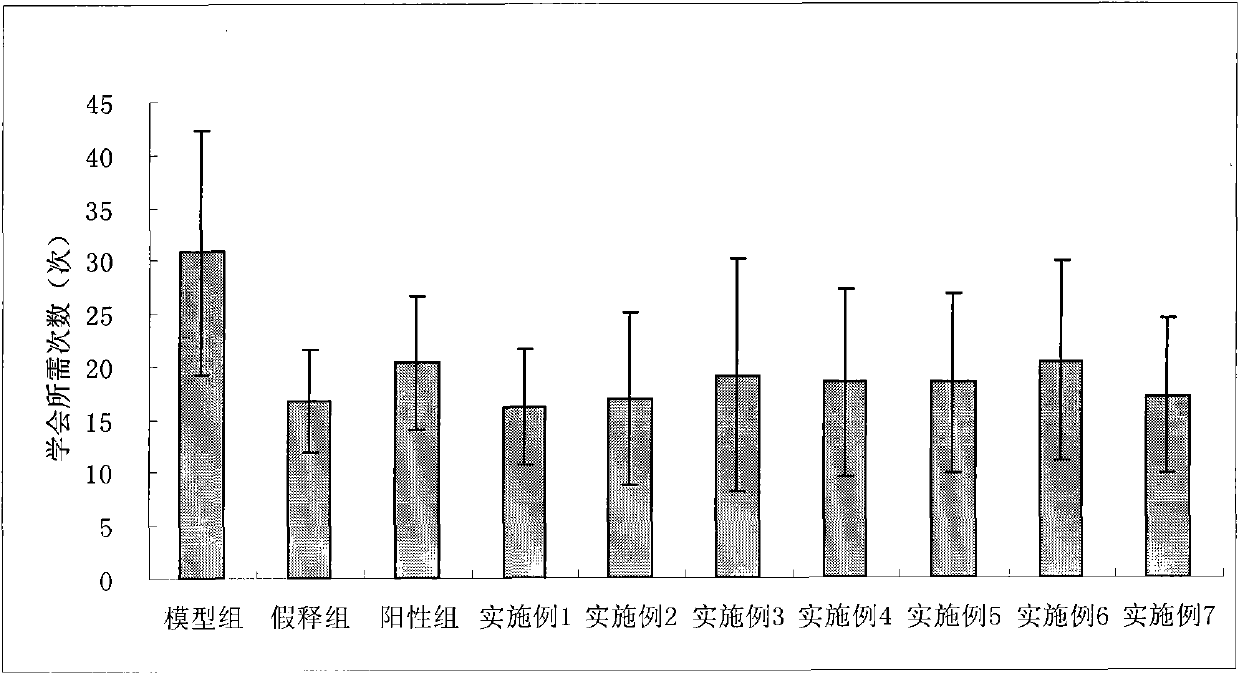
Image
Examples
Embodiment 1
[0063] Example 1: (S)-1-[3,3-Dimethyl-2-oxo-4-(2-piperonyl-5-yl-ethylcarbamoyloxy)butyryl]piperidine-2 -carboxylic acid
[0064] Structure of (S)-1-[3,3-dimethyl-2-oxo-4-(2-piperonan-5-ylethylcarbamoyloxy)butyryl]piperidine-2-carboxylic acid as follows:
[0065]
[0066] 1. Preparation of (S)-piperidine-2-carboxylic acid benzyl ester (compound 2):
[0067] Compound 1 (50 g, 0.39 mol) was dissolved in 500 mL of toluene at room temperature, and p-toluenesulfonic acid (66.7 g, 0.39 mol) and benzyl alcohol (50.5 g, 0.468 mol) were added. After the addition, the reaction was refluxed overnight. After cooling to room temperature, 300 mL of ethyl acetate was added and stirred for 30 minutes. The mixture was then filtered and the filter cake was washed with ethyl acetate (3*100 mL). The filter cake was dissolved in 500 mL saturated aqueous potassium carbonate and extracted with ethyl acetate (2*300 mL). The organic phases were combined and dried over anhydrous sodium sulfate,...
Embodiment 2
[0076] Example 2: 2-[3,3-Dimethyl-2-oxo-4-(2-piperon-5-yl-ethylcarbamoyloxy)butyryl]-1,2,3,4 -Tetrahydroisoquinoline-3-carboxylic acid
[0077] 2-[3,3-Dimethyl-2-oxo-4-(2-piperonyl-5-ylethylcarbamoyloxy)butyryl]-1,2,3,4-tetrahydroisoquinoyl The structure of line-3-carboxylic acid is as follows:
[0078]
[0079] Its preparation method is as follows:
[0080]
[0081] 1.1,2,3,4-tetrahydroisoquinoline-3-carboxylate ethyl ester (compound 7):
[0082] Thionyl chloride (8 mL, 113 mmol) was added dropwise to a solution of compound 6 (10 g, 56.5 mmol) in 100 mL of ethanol at room temperature. After the dropwise addition was completed, it was heated to reflux overnight. Then the solvent was removed and saturated aqueous potassium carbonate was added to pH = 8, and extracted with ethyl acetate (2*100 mL). The organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and then subjected to column chromatography to obtain 10 g of an oily product with a yi...
Embodiment 3
[0089] Example 3: (S)-1-[3,3-Dimethyl-2-oxo-4-(2-piperonyl-5-yl-ethylcarbamoyloxy)butyryl]indoline- 2-Carboxylic acid
[0090] (S)-1-[3,3-Dimethyl-2-oxo-4-(2-piperonyl-5-ylethylcarbamoyloxy)butyryl]indoline-2-carboxylic acid The structure is as follows:
[0091]
[0092] Its preparation method is as follows:
[0093]
[0094] 1. Preparation of (S)-indoline-2-carboxylic acid ethyl ester (compound 11):
[0095] Thionyl chloride (9.1 mL, 122.7 mmol) was added dropwise to a solution of compound 10 (10 g, 61.3 mmol) in 100 mL of ethanol at room temperature. After the dropwise addition was completed, it was heated to reflux overnight. Then the solvent was removed and saturated aqueous potassium carbonate was added to pH = 8, and extracted with ethyl acetate (2*100 mL). The organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain 11.0 g of an oily product with a yield of 94%. Low resolution mass spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com