Organic electroluminescent device comprising triazine derivatives
An electroluminescent device, organic technology, applied in the direction of electric solid-state devices, organic chemistry, luminescent materials, etc., can solve the problems of not being able to vapor phase deposition, not being disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
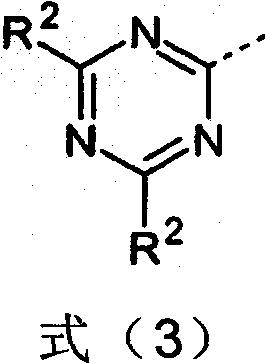
Image
Examples
Embodiment 1
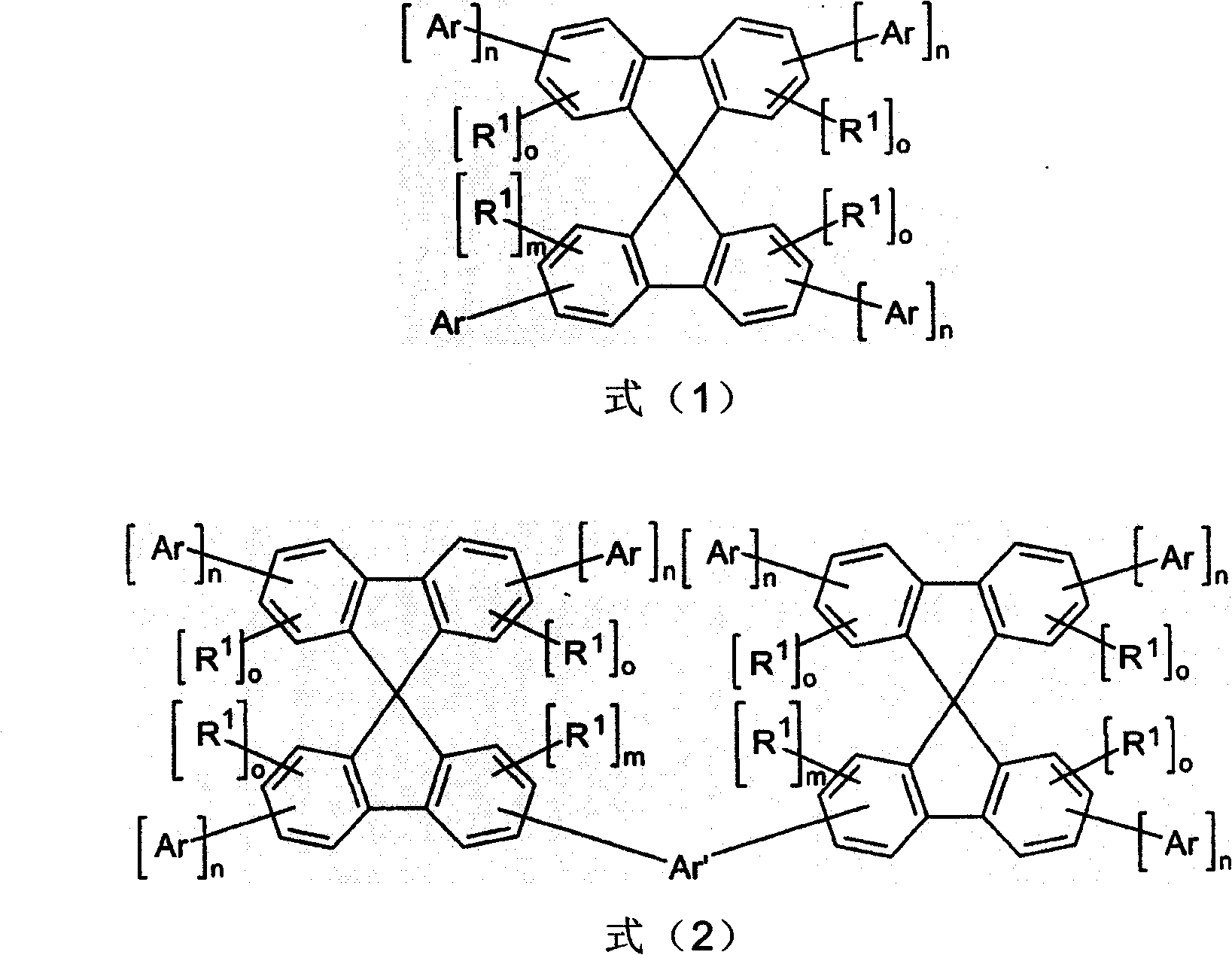
[0152] Example 1: Synthesis of 2-(4,6-bis[3,1'; 5,1"]terphenyl-1-yl)-1,3,5-triazin-2-yl)-spiro-9, 9'-bifluorene
[0153]
[0154] a) Synthesis of 2-chloro-(4,6-bis[3,1′; 5,1″]terphenyl-1-yl)-1,3,5-triazine
[0155] 82.6 ml of a 2.0 molar solution of n-butyllithium in hexane was slowly added dropwise to 50.30 g (163 mmol) of 1-bromo-[3,1′;5,1″]terphenyl cooled to -78°C -1-yl in a solution of 400ml of anhydrous tetrahydrofuran, and the mixture was stirred for 15 minutes.The reaction solution was slowly added dropwise to 10.00g (45mmol) of cyanuric chloride cooled to -78°C in 400ml of anhydrous tetrahydrofuran in solution, and the cooling was removed. When RT had been reached, the precipitated product was filtered off. The yield was 21.08 g (37 mmol), corresponding to 67.9% of theory.
[0156] b) Synthesis of 2-(4,6-bis[3,1′; 5,1″]terphenyl-1-yl)-1,3,5-triazin-2-yl)spiro-9,9′- Bifluorene
[0157] 17.46g (48mmol) of spiro-9,9'-difluorene-2-boronic acid, 21.06g (37mmol) of 2...
Embodiment 2
[0158] Example 2: Synthesis of 2-(4,6-bis(3-([3,1′; 5,1″]terphenyl-1-yl)phen-1-yl)-1,3,5-triazine -2-yl)spiro-9,9'-bifluorene
[0159]
[0160] a) Synthesis of 1-bromo-3-([3,1′; 5,1″]terphenyl-1-yl)phenyl
[0161] Suspend 40.0 g (146 mmol) of 3-boroncarbonyl-[3,1′;5,1″] terphenyl, 18.8 g (146 mmol) of 1-iodo-3-bromobenzene and 109.3 g (730 mmol) of potassium carbonate In 1350ml toluene and 1150ml water.Add 844mg (0.73mmol) tetrakis (triphenylphosphine) palladium (0) in this suspension, and this reaction mixture is heated under reflux 16h.After cooling, the organic phase is separated , washed three times with 200 ml of water, dried over sodium sulfate and then evaporated to dryness. The residue was washed with ethanol, recrystallized from ethyl acetate and finally dried under reduced pressure. The yield was 47.6 g (123 mmol), corresponding to theoretical 84.5%.
[0162] b) Synthesis of 2-chloro-4,6-bis(3-([3,1′;5,1″]terphenyl-1-yl)phen-1-yl)-1,3,5-triazine
[0163] The s...
Embodiment 3
[0166] Example 3: Synthesis of 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-2′,7′-bis([3,1′; 5,1″]- Terphenyl-1-yl)spiro-9,9'-bifluorene
[0167]
[0168] a) Synthesis of 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-2',7'-dibromospiro-9,9'-difluorene
[0169] 47.90g (89mmol) of 2-chlorocarbonyl-2',7'-dibromospiro-9,9'-difluorene, 11.90g (89mmol) of aluminum trichloride and 1.9ml (27mmol) of thionyl Dichloride was suspended in 260ml of dichlorobenzene in a flask. Then 19.3 ml (187 mmol) of benzonitrile were slowly added. The reaction mixture was stirred at 100 °C for 1 h. 9.55 g (179 mmol) of ammonium chloride were added and the batch was stirred at 100° C. for 16 h. After cooling to RT, the reaction solution was added to 3.5 L of methanol, and the mixture was stirred for 45 min. The precipitated solid was filtered off and recrystallized from toluene. Yield 18.8 g (26.7 mmol), corresponding to 2 g.8% of theory.
[0170] b) Synthesis of 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-2′,7′-bis([3,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com