Pharmaceutical agent for promoting functional regeneration of damaged tissue
A technology of tissue regeneration and accelerators, applied in the direction of microorganisms, drug combinations, tissue culture, etc., can solve the problems of poor survival prognosis and insufficient effect of malignant glioma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
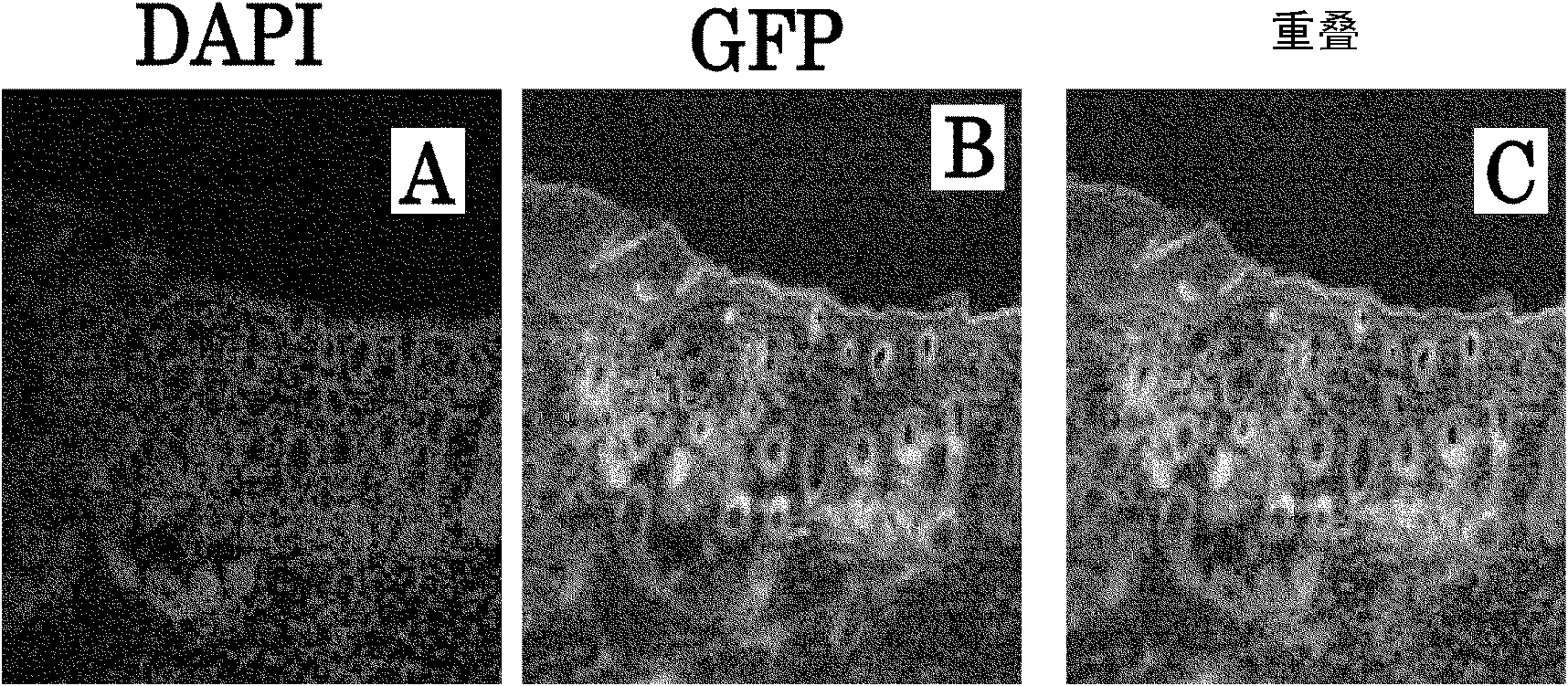
[0168] Objective: To evaluate the involvement of bone marrow-derived cells in the functional regeneration of transplanted skin tissue in vivo
[0169] Method: For the purpose mentioned above, research was carried out by the following method.
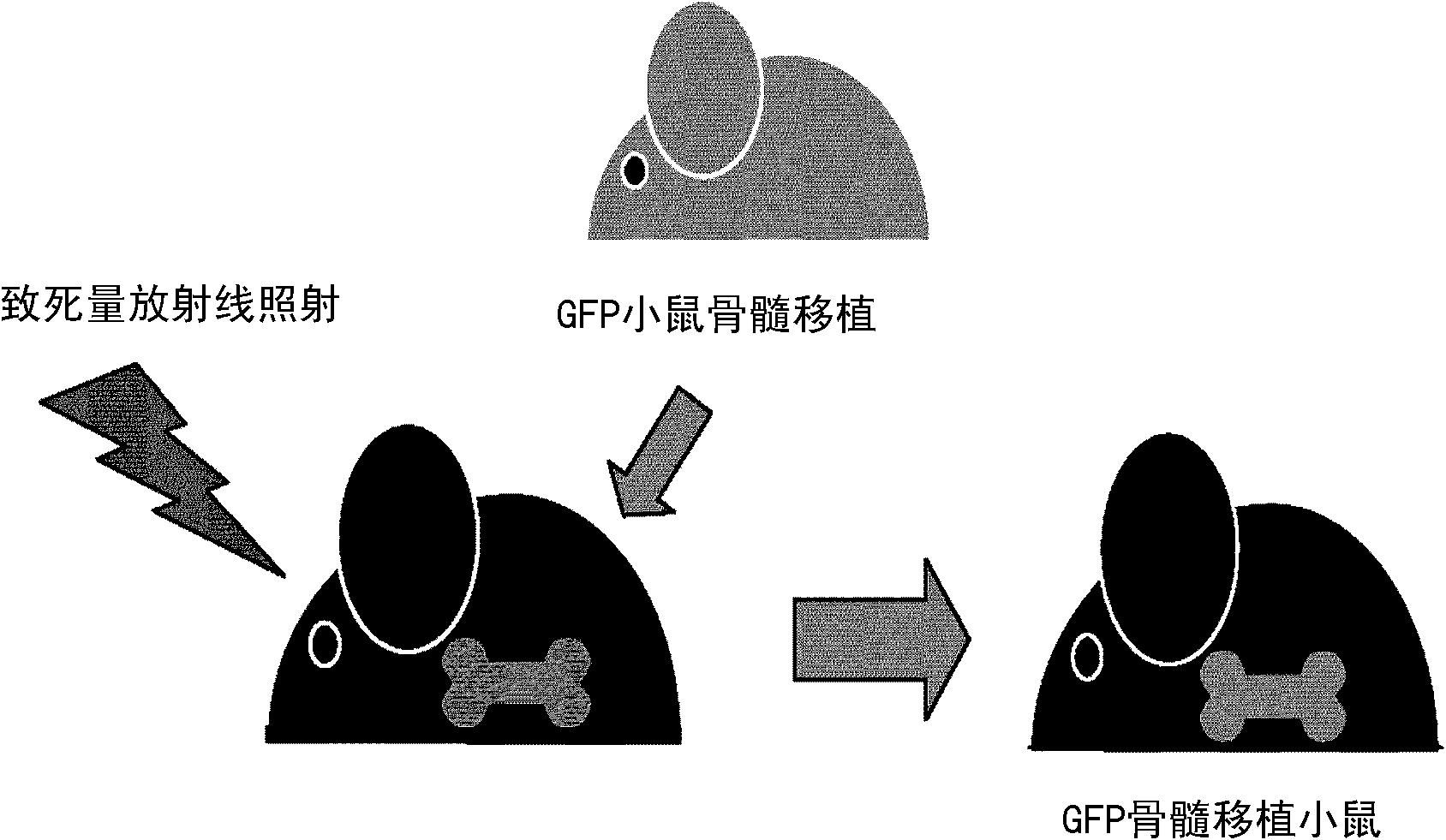
[0170] 1) Using the in vivo skin transplantation system implanted into GFP bone marrow transplanted mice, the degree of participation of bone marrow-derived cells in the functional regeneration of the grafted skin was studied. Specifically, C57BL / 6 male mice (6-8 weeks old) were irradiated with a lethal dose of radiation (10Gy), and then transplanted bone marrow cells (5×10 6 each / 0.1ml physiological phosphate buffer solution pH7.4)( figure 1 ).
[0171] 2) After transplanted bone marrow cells were implanted (6 weeks), newborn mouse skin (female) was transplanted on the back skin of the obtained GFP bone marrow transplanted mouse.
[0172] 3) After the grafted skin is implanted and the skin tissue is fully regenerated (4 weeks), the c...
Embodiment 2
[0178] Objective: To identify bone marrow-derived tissue stem cell-inducing factors in skin tissue extracts
[0179]METHODS: In order to identify the bone marrow mesenchymal stem cell mobilization factors presumed to be released from the resected skin in a state of hypoperfusion, the following study was carried out.
[0180] 1) To obtain mouse bone marrow-derived mesenchymal stem cells, mouse bone marrow cells were collected from the femur or lower leg bone of a C57BL / 6 mouse, and D-MEM (manufactured by Nacalai) containing 10% fetal bovine serum was used as The cell culture medium was added to the cell culture dish, and cultured at 37° C. and a carbon dioxide gas concentration of 5%. When the cells proliferate to an area of 70-100% relative to the bottom area of the culture dish, the cells are detached from the culture dish with 0.25% trypsin 1mM EDTA (manufactured by Nacalai), and then subcultured under the same conditions. to cultivate. The subculture operation was rep...
Embodiment 3
[0192] Objective: To confirm the therapeutic effect of S100A8 on skin ulcer in normal mice and diabetic mice
[0193] Method: The recombinant S100A8 protein was given to the mouse skin ulcer model to study the effect of ulcer treatment. As the test mice, C57 / B16 mice transplanted with GFP-expressing bone marrow cells or diabetic model mice BKS.Cg-m+ / +Leprdb / J (db mice) were used. Skin ulcers with a diameter of 6 mm were made on the mouse skin. The skin around the skin defect portion of the mouse-made skin ulcer shrank rapidly. In this experiment, in order to create a model in which the defective skin does not shrink but is treated by covering it with regenerated skin, a silicone rubber disc with an outer diameter of 10 mm, an inner diameter of 6 mm, and a thickness of 0.5 mm was used for skin surgery. Adhesive (Aron alpha A) and nylon thread are fixed to the skin around the ulcer. Then, the recombinant S100A8 protein was directly administered to the ulcer surface at 1.5 μg / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com