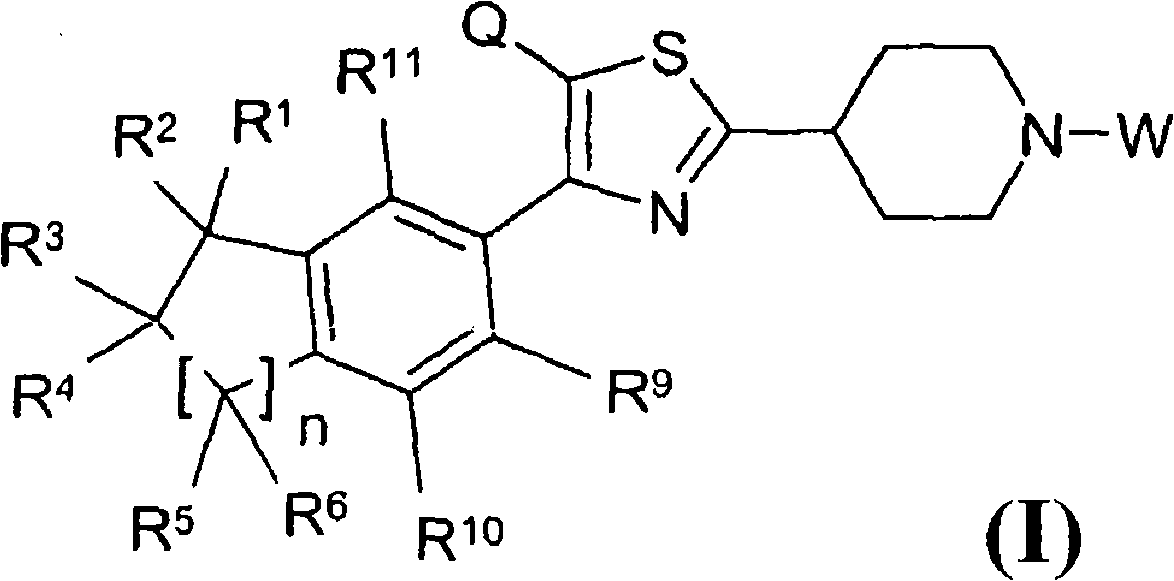
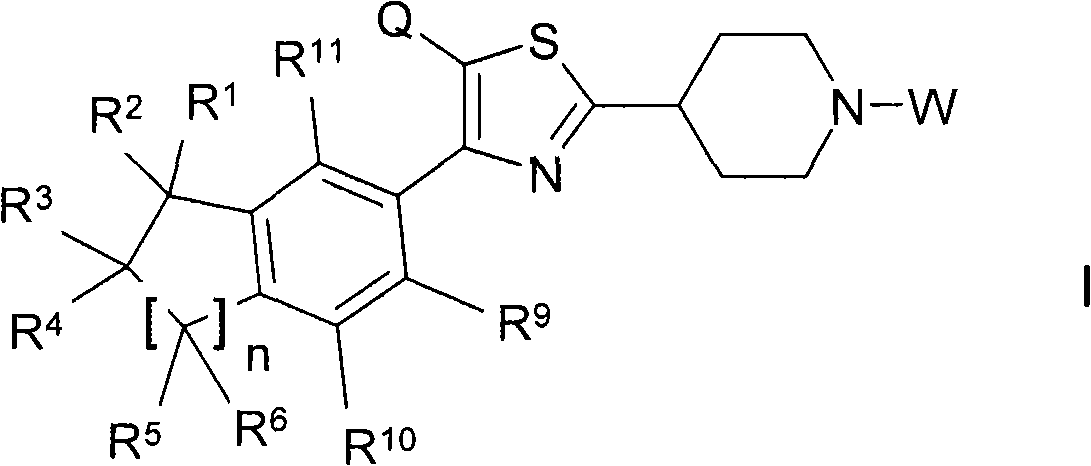
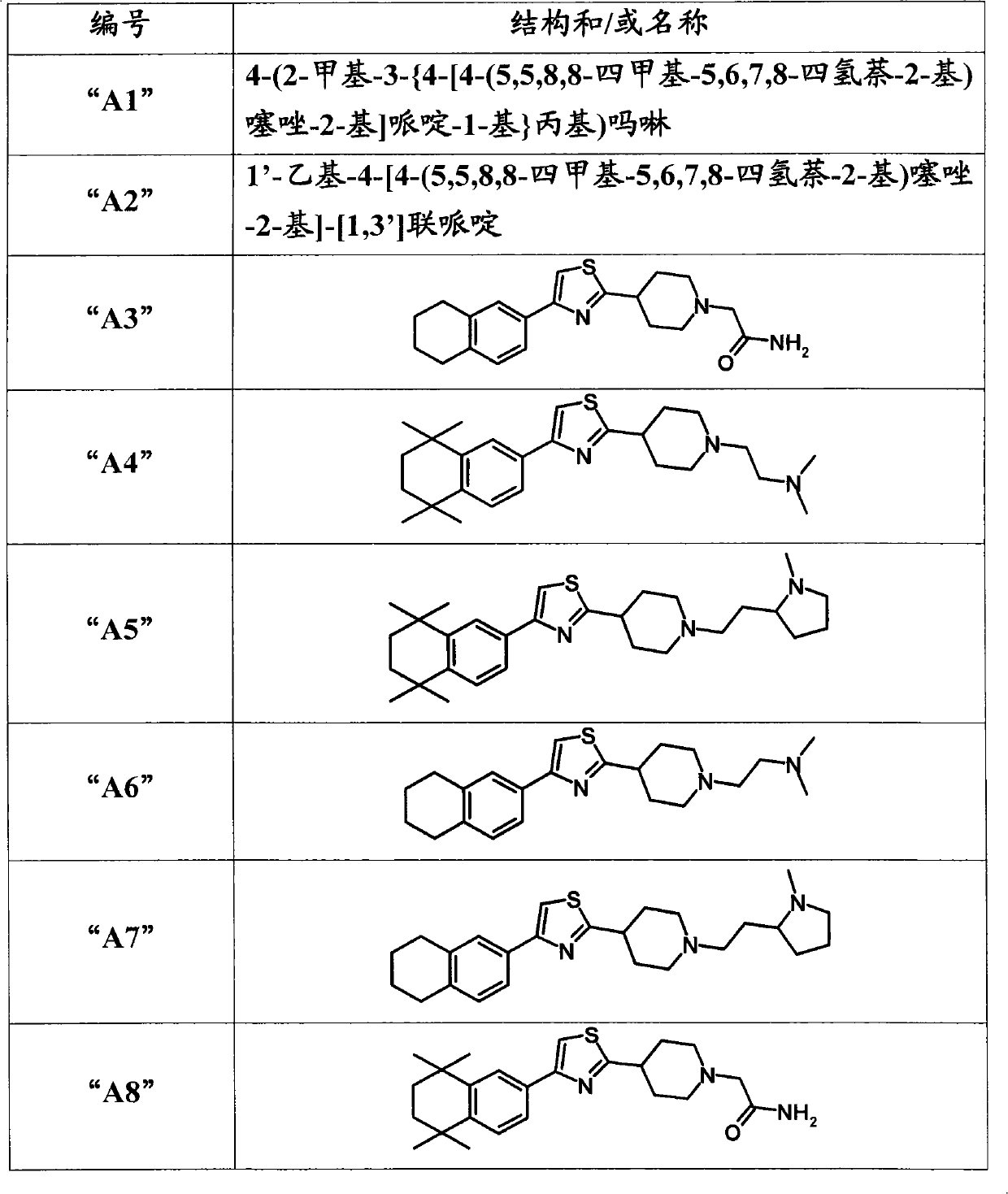
Thiazolyl piperdine derivatives
A technology of groups and compounds, applied in the field of thiazolylpiperidine derivatives, can solve problems such as failure to identify interaction partners
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0361] 4-(2-Methyl-3-{4-[4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)thiazol-2-yl ]Piperidin-1-yl}propyl)morpholine ("A1") Preparation
[0362]
[0363] 200 mg (0.46 mmol) of 4-[4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)thiazol-2-yl]piperidine hydrogen Bromate with 122 mg (0.69 mmol) of 4-(3-chloro-2-methyl-propyl)morpholine in 5 mL of ethanol and 320 μL (2.3 mmol) of triethylamine, in microwave, irradiated at 160 °C 2 hours. The reaction mixture was evaporated and purified by silica gel column chromatography. The product was further purified by preparative HPLC.
[0364] Yield: 49 mg of "A1" trifluoroacetate salt, pale yellow oil; ESI: 496 g / mol [M+H], HPLC: Rt.=1.94 min.
Embodiment 2
[0366] 1'-Ethyl-4-[4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)thiazol-2-yl]-[1, Preparation of 3'] Bipiperidine ("A2")
[0367]
[0368] 500 mg (1.41 mmol) of 4-[4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)thiazol-2-yl]piperidine were mixed with 210 mg (1.42 mmol) of 3-chloro-1-ethylpiperidine, 360 mg (4.29 mmol) of sodium bicarbonate, and 210 mg (1.40 mmol) of sodium iodide were suspended in 40 mL of DMF and placed in a microwave at 120 Irradiated for 6 hours at °C. The reaction mixture was evaporated and the residue was dissolved in ethyl acetate and 0.1N NaOH. The organic phase was separated, evaporated and purified by silica gel column chromatography.
[0369] Yield: 45 mg of "A2" hydrochloride, brown crystals, ESI: 466 g / mol [M+H];
[0370] 1 H NMR (500MHz, DMSO-d 6 ) δ [ppm] 11.19(b, 1H), 7.93(s, 1H), 7.65(s, 2H), 7.11(d, J=8.4, 1H), 3.0-4.0 (overlap, 7H), 2.76(d, J=19.6, 4H), 2.15-2.45 (b, 5H), 1.85-2.10 (m, 4H), 1.73-1.82 (m, 4H), 1...
Embodiment 3
[0381] (2-{4-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)thiazol-2-yl]piperidin-1-yl Preparation of} ethyl) tert-butyl carbamate ("A37")
[0382]
[0383] 10 mL of THF and 200 μL of glacial acetic acid were added to 200 mg (0.46 mmol) of 4-[4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl] ) thiazol-2-yl]piperidine hydrobromide, and 154 mg (0.92 mmol) of tert-butyl (2-oxoethyl)carbamate was added. 195 mg (0.92 mmol) of sodium triacetoxyborohydride were then added and the reaction mixture was stirred at room temperature for 24 hours. The reaction mixture was filtered, the mother liquor was evaporated and the residue was purified by preparative HPLC to give "A37";
[0384] ESI: 498 (M+H), HPLC: 3.34 min.
[0385] The following compounds were prepared analogously to the methods described above:
[0386]
[0387]
[0388]
[0389]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com