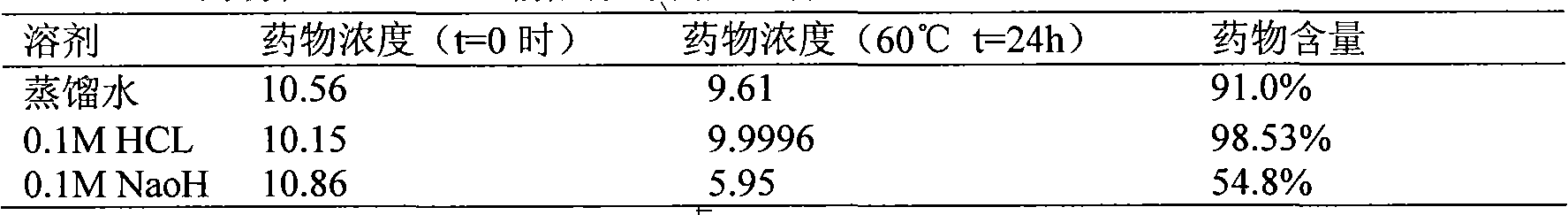
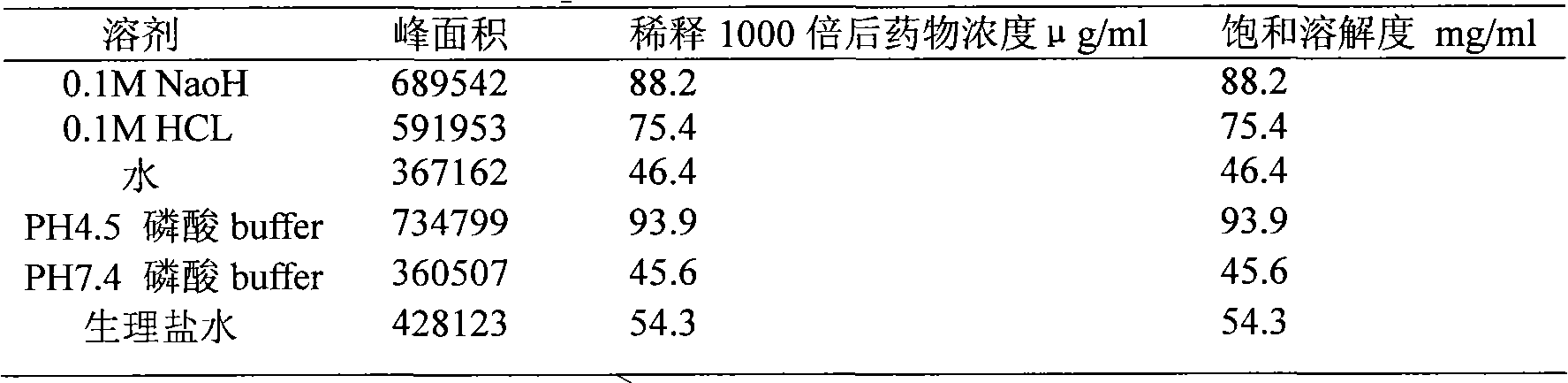
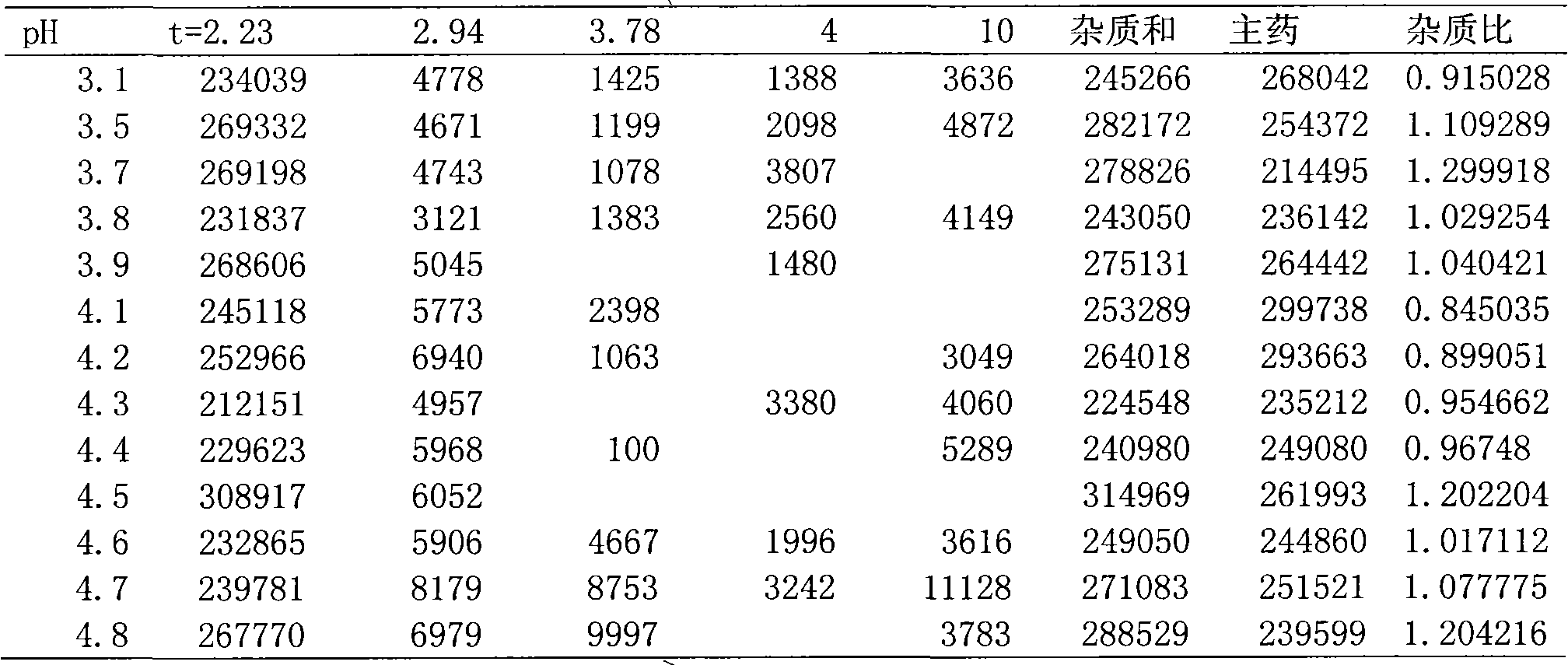
Preparation containing 1-(cinnamoyl)-4-(cyclopentylamine acetyl) piperazidine and/or salt thereof and pharmaceutic adjuvant
A technology of amylamine acetyl group and cinnamoyl group, applied in the field of medicine, can solve the problems of increased content of cis-isomer of cinnamate maleate, great influence on drug safety, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] One of the most preferred prescriptions and processes for solution injections:
[0043] 1. Prescription:
[0044] 1-(cinnamoyl)-4-(cyclopentylaminoacetyl)piperazine maleate 40g
[0045] D-Sorbitol 25.2g
[0046] Disodium hydrogen phosphate 2.5g
[0047] Add water for injection to 1000ml
[0048]
[0049] A total of 500 sticks were prepared
[0050]2. Process: Add D-sorbitol into 75% water for injection, dissolve within 1 minute at 60°C, then add the main drug to the above solution until it is partially dissolved, add 3 / 4 of disodium hydrogen phosphate to completely dissolve the main drug , slowly add the remaining disodium hydrogen phosphate solution, adjust the pH to 4.1; then add 0.1% needle-shaped activated carbon, keep stirring at 60°C for 15 minutes, filter while it is hot, and adjust the volume to the full amount; the intermediate product is obtained, and its pH is measured , if not within 3....
Embodiment 2
[0057] 1. Prescription
[0058] 1-(cinnamoyl)-4-(cyclopentylaminoacetyl)piperazine maleate 30g
[0059] D-Sorbitol 27.5g
[0060] Disodium hydrogen phosphate 2.4g
[0061] Add water for injection to 1000ml
[0062]
[0063] A total of 500 sticks were prepared
[0064] 2. Preparation process: Reference Example 1
Embodiment 3
[0066] 1. Prescription
[0067] 1-(cinnamoyl)-4-(cyclopentylaminoacetyl)piperazine maleate 35g
[0068] D-Sorbitol 26.8g
[0069] Disodium hydrogen phosphate 2.47g
[0070] Add water for injection to 1000ml
[0071]
[0072] A total of 500 sticks were prepared
[0073] 2. Preparation process: Reference Example 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com