Standard fingerprint spectrum of Chinese medicinal composition, and measurement method and application thereof
A standard fingerprint and determination method technology, applied in the field of traditional Chinese medicine analysis, can solve the problem that traditional Chinese medicine has no mandatory requirements, and achieve the effect of objective conclusion and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: Fingerprint Spectrum Determination
[0085] The preparation method of capsule is as follows:
[0086] Prescription: Chuanxiong 784g Gastrodia elata 196g
[0087] Preparation method: the above two flavors are crushed, mixed, extracted twice with 90% ethanol under reflux, each time for 2 hours, the extracts are combined, filtered, and the filtrate is recovered from ethanol and concentrated into clear ointment I with a relative density of 1.27 to 1.28; The slag is decocted twice with water, each time for 1 hour, the decoction is combined, filtered, the filtrate is concentrated into clear paste II with a relative density of 1.27-1.28, the above clear paste I and clear paste II are combined, an appropriate amount of silicon dioxide is added, vacuum Dry, pulverize, sieve, pack into capsules, make 1000 capsules, and get ready. Specifications: 0.34g per capsule
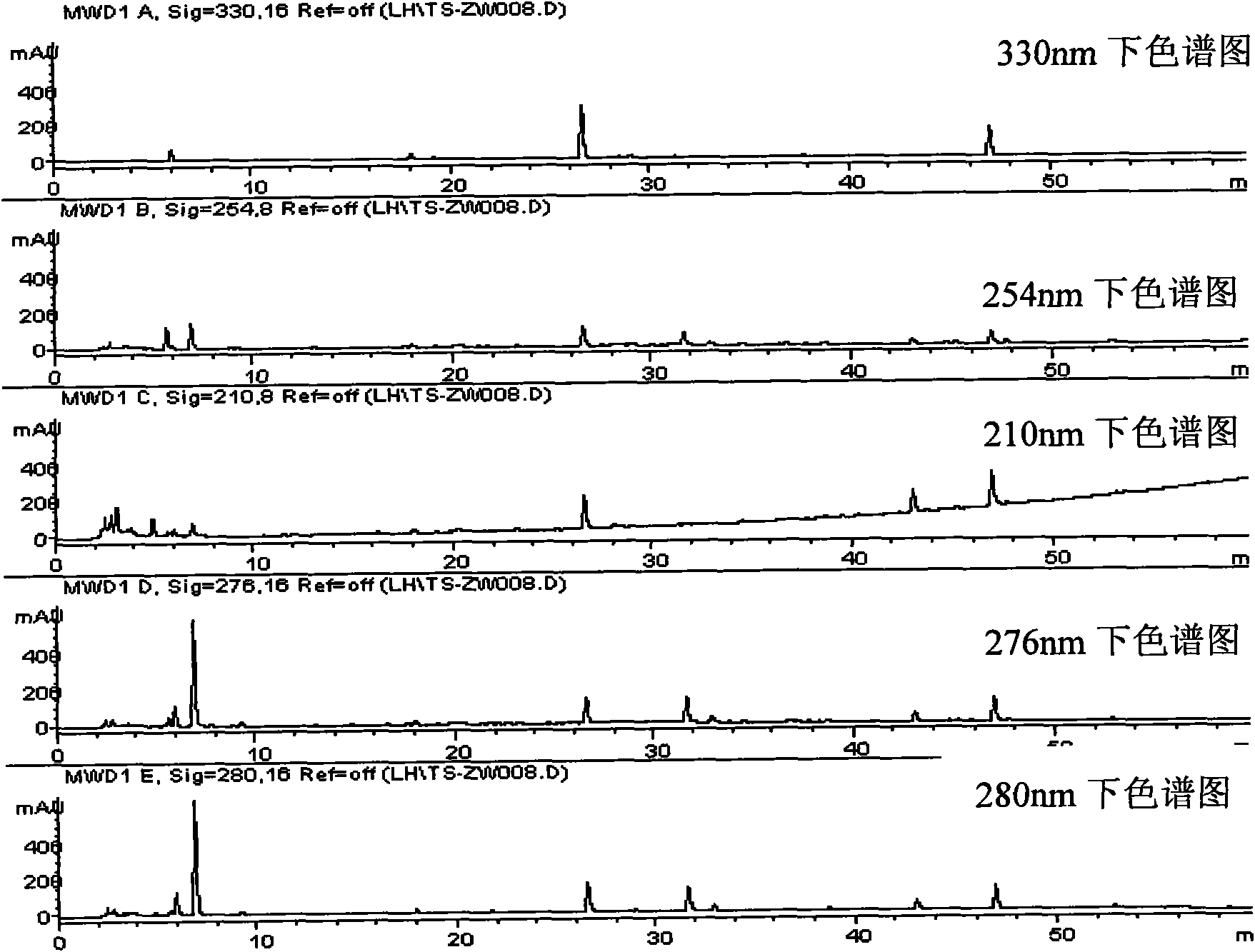
[0088] Fingerprint detection:
[0089] Take the contents of this product, mix well, grind finely, ...
Embodiment 2
[0095] Embodiment 2: Obtaining of standard fingerprint spectrum.
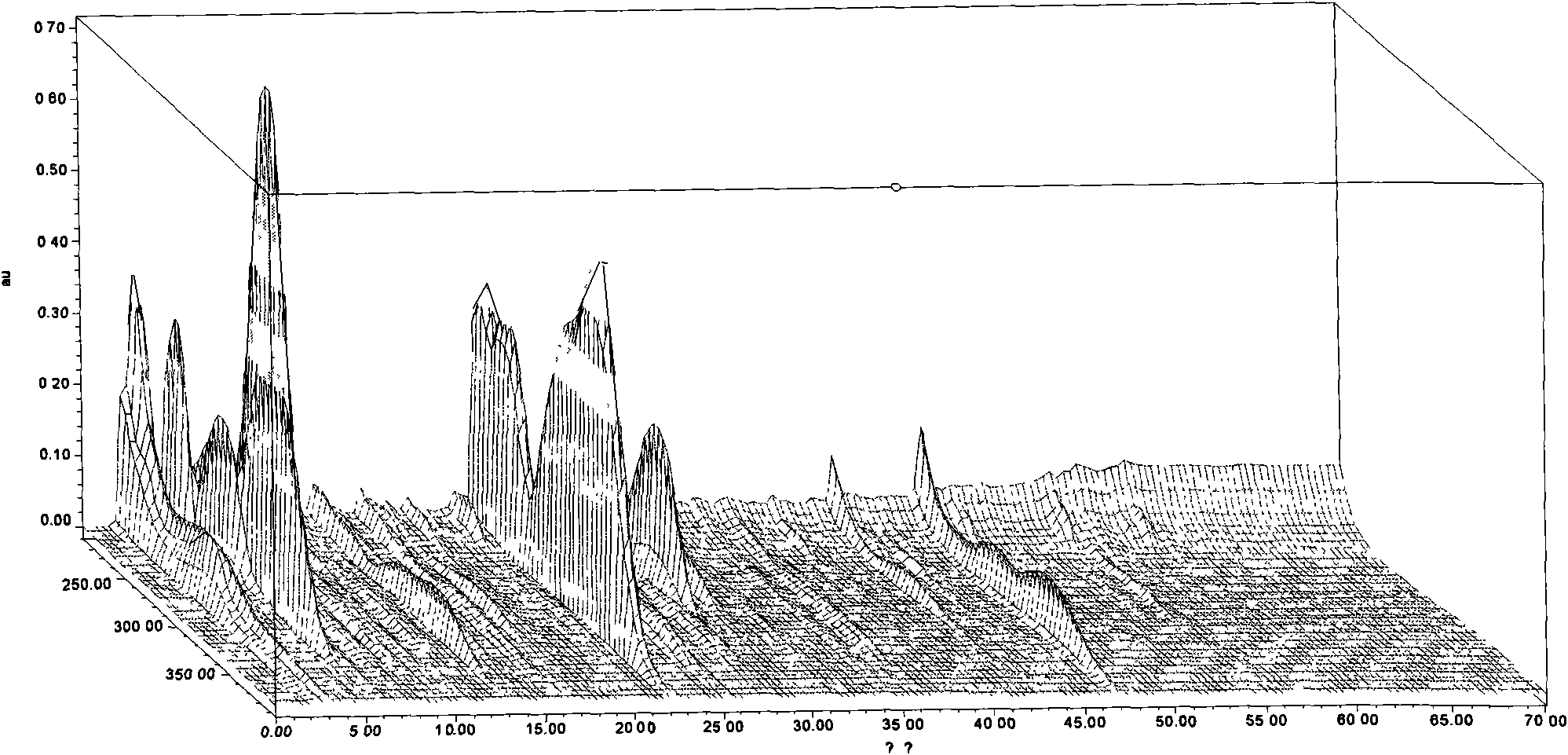
[0096] 10 batches of this Chinese medicine composition capsule finished product, prepare need testing solution by the preparation method of need testing solution, measure according to embodiment 1 method (detection wavelength is 276nm), result calculation similarity, the results are shown in Table 6, with similarity software Based on the fingerprints of these 10 batches of test products, the "common pattern" was obtained as a standard fingerprint. The results are shown in image 3 .
[0097] Table 6 Similarity of Ten Batches of Capsule Finished Products of the Composition (Wavelength 276nm)
[0098] batch number
Embodiment 3
[0099] Embodiment 3: the determination of product quality control standard
[0100] The similarity evaluation system for chromatographic fingerprints of traditional Chinese medicines stipulated by the National Pharmacopoeia Commission was adopted, and the version was the 2004 edition.
[0101] The fingerprints and standard fingerprints of the 10 batches of finished products described in Example 2 were used to calculate the similarity, and the fingerprints and standard fingerprints of the traditional Chinese medicine composition were calculated by similarity software, and the similarity was not less than 0.80.
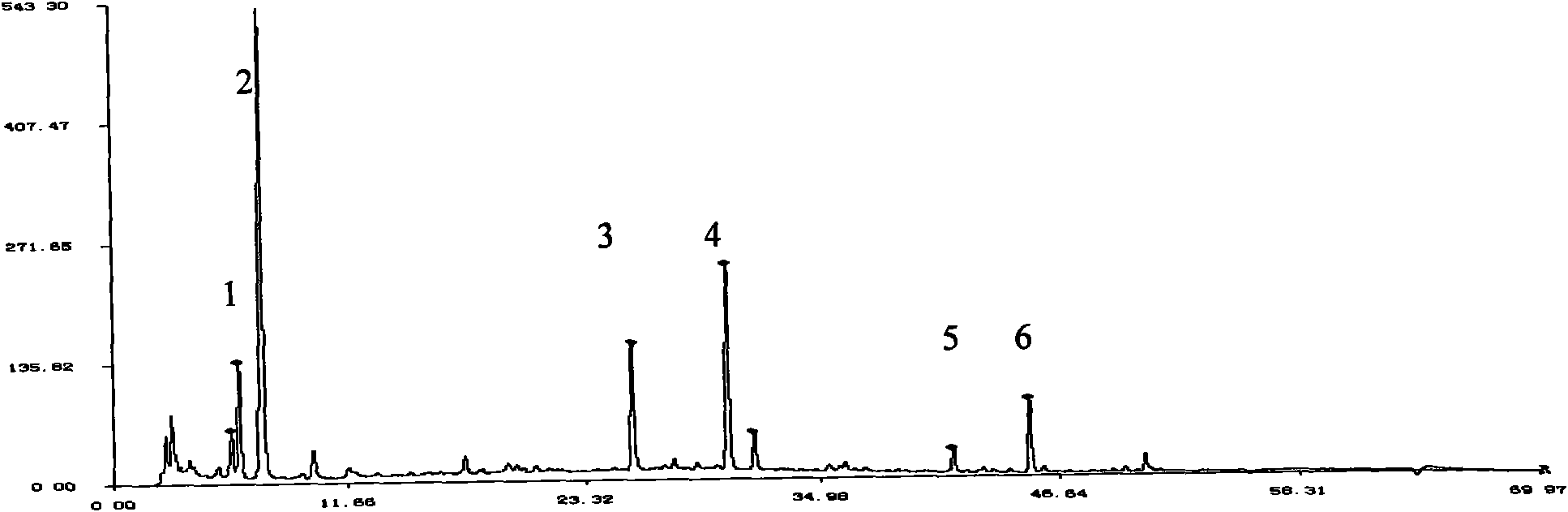
[0102] The main characteristics of the fingerprint of the product quality control standard:
[0103] Present 6 main chromatographic peaks in this collection of figures, wherein No. 3 peak is the identical chromatographic peak with the peak retention time of reference material ferulic acid, compared with No. 3 peak retention time (by 1), other 5 chromatographic peaks The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com