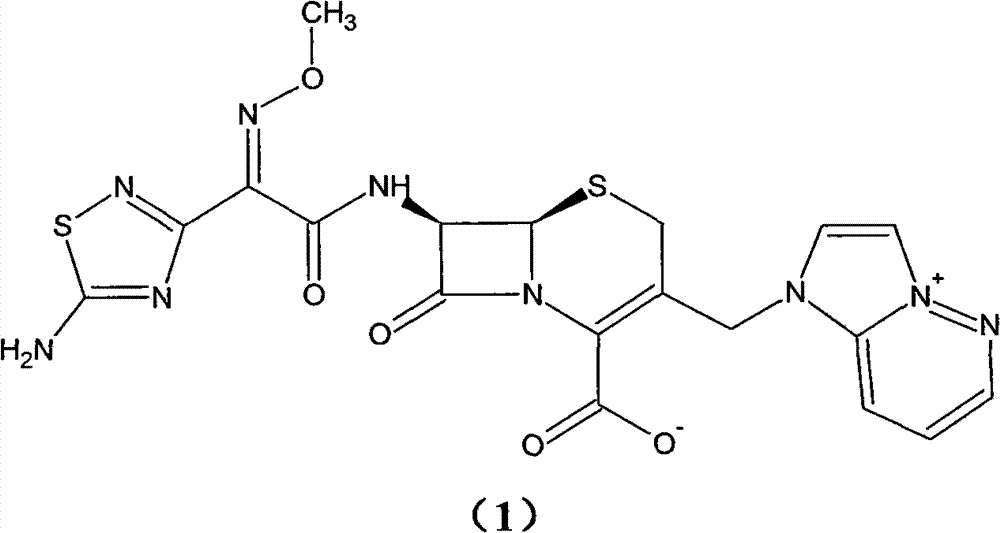
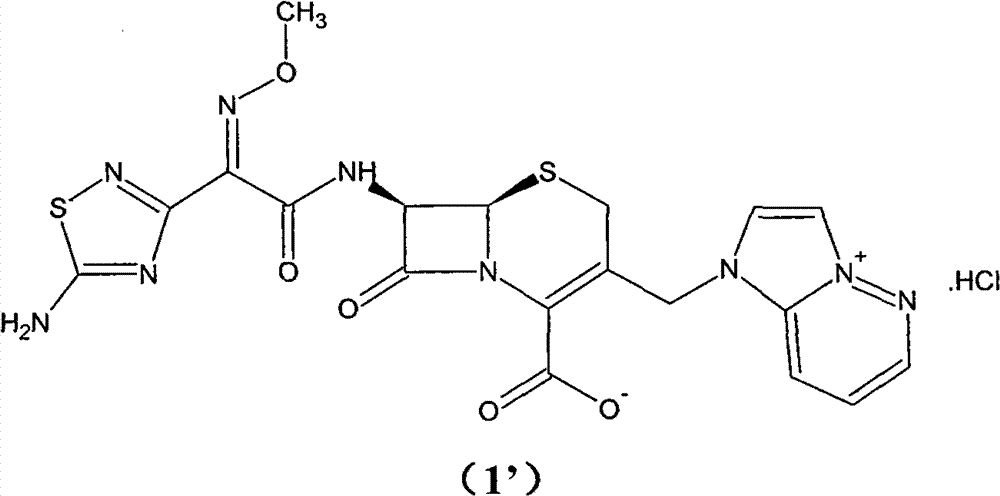
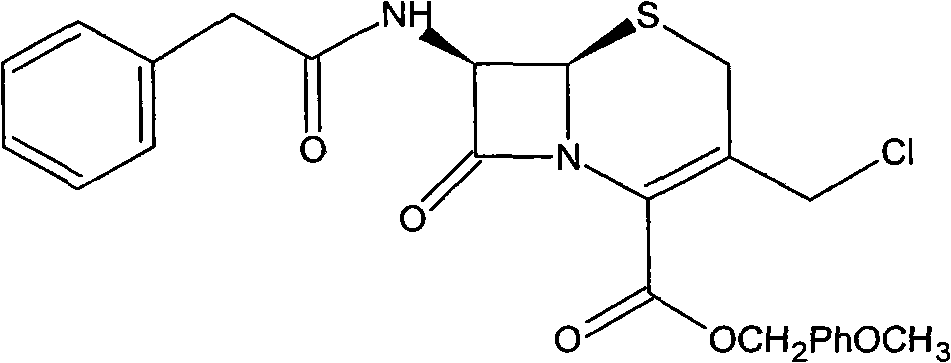
Preparation method of cefozopran hydrochloride
A technology of cefazolam hydrochloride and hydrochloric acid, applied in the field of pharmaceutical antibiotics, can solve the problems of unfavorable continuity, large-scale industrial production, low cefazolam yield, long reaction time, etc., and achieves elimination of discoloration phenomenon, excellent quality, The effect of low product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) GCLE12.5g, NaI4.1g, acetone 80ml at 30°C, stirred for 15 minutes. Then, 4.5 g of imidazopyridazine was added, the temperature was raised to 35° C., and the mixture was stirred for 5 hours. Add 125 ml of isopropanol, and stir for 30 minutes after addition. Cool down to 0° C., stir for 1 hour, filter, and wash the filter cake twice with a mixture of 50 ml of isopropanol and 30 ml of acetone. Drying in vacuo gave a yellow-green solid (GIMPE). Add 25 ml of phenol, raise the temperature to 50°C, and stir for 10 hours. Cool down to 40°C, add 10ml of isopropyl ether and 5ml of isopropanol mixture, and then stir for 20 minutes. The reaction solution was added to 60ml of isopropyl ether, and a yellow solid was precipitated. After stirring for 60 minutes, it was filtered, and the filter cake was washed with 30ml of acetone and 40ml of isopropyl ether. The filter cake was added into a mixture of 1.2 g of sodium bicarbonate, 45 ml of water, and 10 ml of acetone and stirred ...
Embodiment 2
[0067] (1) 13g of GCLE, 4.1g of NaI, 80ml of acetone were stirred at 30°C for 15 minutes. Then, 4.5 g of imidazopyridazine was added, the temperature was raised to 35° C., and the mixture was stirred for 7 hours. Add 130 ml of isopropanol, and stir for 30 minutes after addition. Cool down to 0° C., stir for 1 hour, filter, and wash the filter cake twice with 50 ml of isopropanol. Drying in vacuo gave a yellow-green solid (GIMPE). Add 25 ml of phenol, raise the temperature to 50°C, and stir for 10 hours. Cool down to 40°C, add 10ml of isopropyl ether and 5ml of isopropanol mixture, and then stir for 20 minutes. The reaction liquid was added into 60ml of isopropyl ether, and a yellow solid was precipitated. After stirring for 60 minutes, it was filtered, and the filter cake was mixed and washed with 40ml of isopropyl ether. The filter cake was added to a mixture of 1 g of sodium bicarbonate, 45 ml of water, and 10 ml of acetone and stirred to dissolve. Add 10ml of isopropyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com