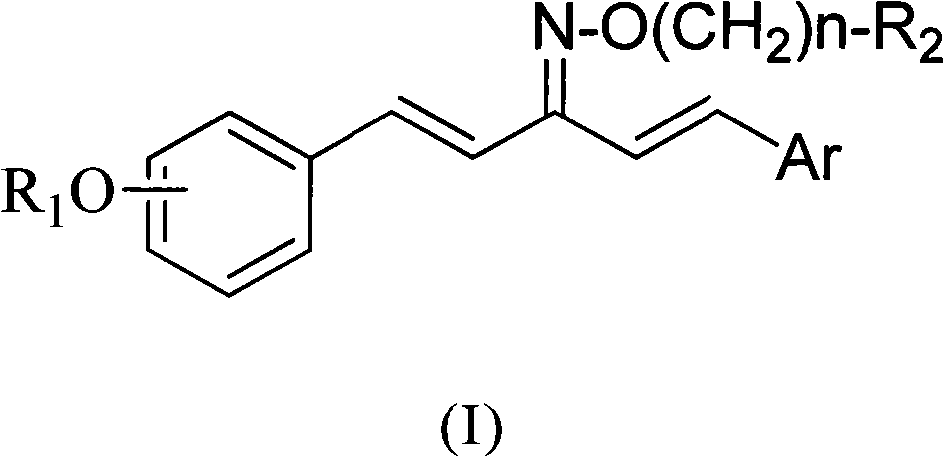
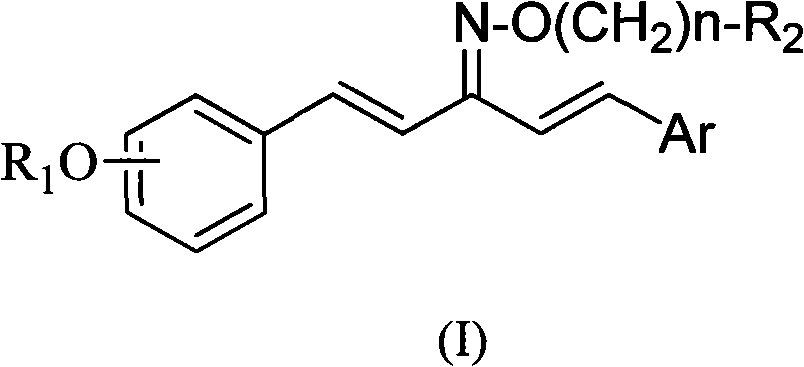
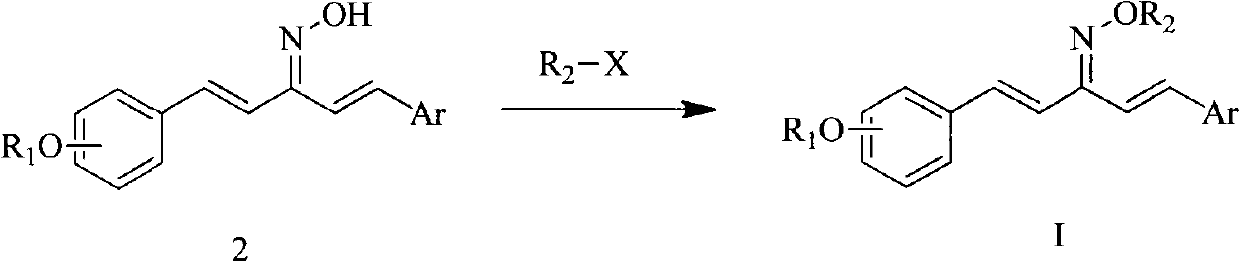1,5-disubstituted aryl-1,4-pentadiene-3-ketoxime ether compound and preparation method thereof and insecticidal activity application
A compound, pentadiene technology, applied in the field of pesticides, can solve problems such as uninvolved, and achieve the effects of good control effect and good insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1, 2-chloro-5-methylpyridine-1-(2-benzyloxyphenyl)-5-(4-methoxyphenyl)-1,4-pentadien-3-one oxime Ether (compound number is A):
[0101] (1) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one
[0102] Add 2-hydroxybenzaldehyde (20.0mmol), 24mL acetone, and 10mL water successively in a 100mL three-necked flask, stir at room temperature until the solids are completely dissolved, and add a solution of sodium hydroxide (24mmol)+20mL water drop by drop, and the reaction solution consists of light The yellow clear liquid turns into a wine-red clear liquid, the reaction is complete, the acetone is removed by rotary evaporation, 70mL hot water is added to the residual liquid until the red solid is completely dissolved, and carbon dioxide is introduced for about 30 minutes until the reaction liquid no longer changes color, and a light yellow solid appears , filtered, washed with water, dried, and recrystallized with acetone / water to obtain a light yellow solid with a yield o...
Embodiment 2
[0111] Example 2. Benzyl-1-[2-(4-methoxy)benzyloxyphenyl]-5-(4-methoxyphenyl)-1,4-pentadiene-3-one oxime Ether (compound number is D):
[0112] (1) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one
[0113] Synthesize as in Example 1 (1) method and conditions.
[0114] (2) Synthesis of 1-(2-hydroxyphenyl)-5-(4-methoxyphenyl)-1,4-pentadien-3-one
[0115] Synthesize as in Example 1 (2) method and conditions.
[0116] (3) Synthesis of 1-[2-(4-methoxy)benzyloxyphenyl]-5-(4-methoxyphenyl)-1,4-pentadien-3-one
[0117] Into a 250mL three-necked flask, add 1-(2-hydroxyphenyl)-5-(4-methoxyphenyl)-1,4-pentadien-3-one (23mmol), potassium iodide (0.23mmol) in sequence , potassium carbonate (26mmol), 100mL acetone, stirred at room temperature, then added dropwise 4-methoxybenzyl chloride (26mmol), heated to reflux, when the color of the solution changed from red to dark yellow, the reaction was complete, filtered while hot to remove excess Potassium iodide and potassium carbonate, and th...
Embodiment 3
[0123] Embodiment three, 4-methoxybenzyl-1-(4-benzyloxyphenyl)-5-(4-methoxyphenyl)-1,4-pentadiene-3-ketoxime ether ( Compound number is N):
[0124] (1) Synthesis of 4-(4-hydroxyphenyl)-3-buten-2-one
[0125] Add 4-hydroxybenzaldehyde (20.0mmol), 24mL acetone, and 10mL water successively in a 100mL three-necked flask, stir until the solids are completely dissolved, then add a solution of sodium hydroxide (24mmol)+20mL water dropwise, and the reaction solution changes from light yellow to The clear liquid turns into a wine-red clear liquid, the reaction is complete, the acetone is removed by rotary evaporation, 70mL of hot water is added to the residual liquid until the red solid is completely dissolved, and carbon dioxide is introduced for about 30 minutes until the reaction liquid no longer changes color, and a light yellow solid is produced. Filtrate, wash with water, dry, and recrystallize with acetone / water to obtain a light yellow solid with a yield of 84% and a melting ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com