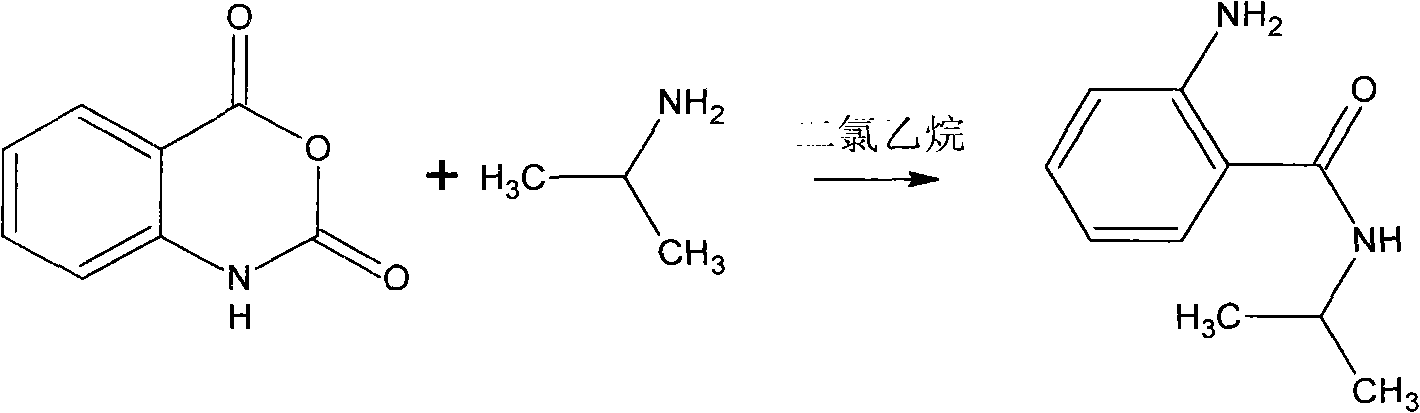
Synthesis method of bentazone midbody 2-amino-N-isopropylbenzamide
A technology of isopropyl benzamide and synthesis method, which is applied in the field of synthesis of bentazone intermediate 2-amino-N-isopropyl benzamide, can solve the problem of large investment, large loss of isatoic anhydride, and difficulty in feeding and other issues to achieve the effect of reducing production costs, reducing production processes, and reducing labor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1) Put 2800L recovered dichloroethane in the static tank for 1 to 2 hours, and add it into the 5000L reactor equipped with anchor stirring to measure the water content to be 0.3% after water separation;
[0016] 2) Add 900 kilograms of water containing 35% undried isatoic anhydride in the reactor under stirring;
[0017] 3) Heating the reaction system to 50-52° C., and evenly adding 300 kg of isopropylamine dropwise within 3 hours. After dripping, keep the reaction temperature and continue to stir for 1 hour, then add 300 kg of water in two times to wash, divide the water, remove part of dichloroethane, and obtain an amide solution with a content of about 25% and a yield of about 93%. The follow-up production process of grass pine.
Embodiment 2
[0019] 1) Put 2800L of recovered dichloroethane in the static tank for 1 to 2 hours, measure the water content of 0.5% after water separation, and add it to the 5000L reactor equipped with anchor stirring;
[0020] 2) Add 900 kilograms of water in 25% undried isatoic anhydride in the reactor under stirring;
[0021] 3) The reaction system was heated to 56-60° C., and 300 kg of isopropylamine was evenly added dropwise within 3 hours. After dripping, keep the reaction temperature and continue to stir for 1 hour, then add 300 kg of water in two times to wash, divide the water, remove part of dichloroethane, and obtain an amide solution with a content of about 20% and a yield of about 94%. The follow-up production process of grass pine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com