Mixed suspension injection liquid containing tylosin and preparation method thereof
A technology for suspension injection and tylosin, which is applied in the field of tylosin-containing suspension injection and its preparation, can solve the problems of few reports and few manufacturers of long-acting preparations, and achieves biocompatibility Good performance, simple and easy preparation process, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: 20% Tylosin Suspension Injection
[0016] [Prescription] Tylosin 20% (W / V)
[0017] Sodium Carboxymethyl Cellulose 0.5% (W / V)
[0018] Tween-80 0.1% (W / V)
[0019] Sodium citrate 0.5% (W / V)
[0020] 1,2-Propanediol 10% (W / V)
[0021] 1N NaOH to pH 7.0
[0022] Methylparaben 0.18% (W / V)
[0023] Propylparaben 0.02% (W / V)
[0024] Add water for injection to 100% (V / V)
[0025] 【preparation】
[0026] Swell and dissolve sodium carboxymethylcellulose, Tween-80, and sodium citrate with water; add the prescribed amount of tylosin tartrate, stir and dissolve; slowly add 1N NaOH dropwise to pH 7.0 to obtain a white milky solution, Stir at the same time; add other additives in the prescribed amount, add water to the full amount, and obtain Tylosin Suspension Injection.
Embodiment 2
[0027] Embodiment 2: Quality Research and Application Test of Tylosin Suspension Injection of the Present Invention
[0028] 1. Quality evaluation
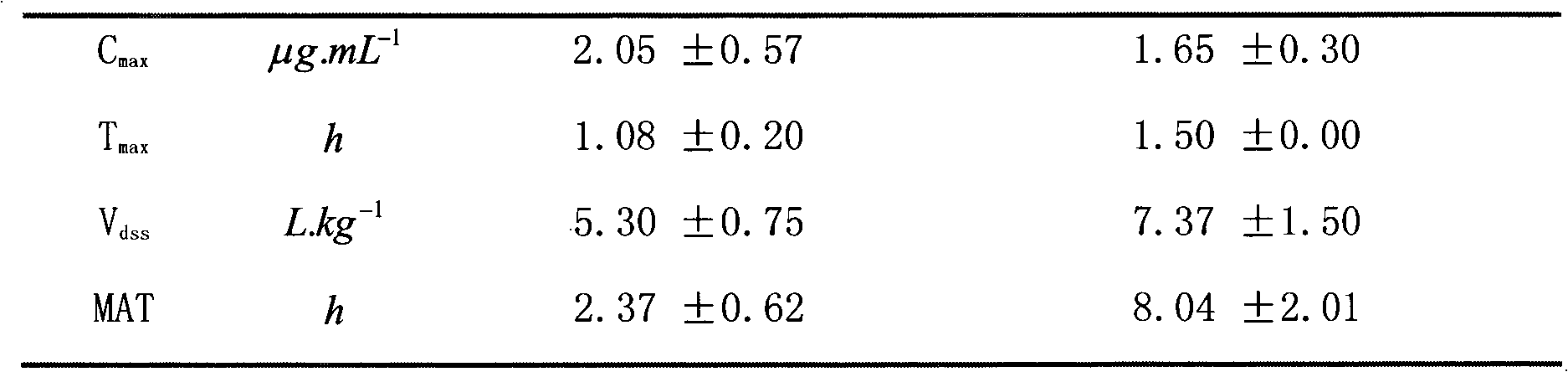
[0029] The tylosin suspension of the present invention has no delamination after being placed for 30 minutes, and the sedimentation volume ratio after being placed for 3 hours is 91%, indicating that the sedimentation speed of the suspension is relatively slow; the re-dispersibility test results show that the suspension is easily dispersed after shaking , no agglomeration; 86.9% of drug particles in the suspension have particle sizes between 2.5 and 15 μm; 8.8% are larger than 15 μm; they meet the requirements for suspension injections.
[0030] 2. Stability test
[0031] Under the conditions of (4500 ± 500) LX strong light irradiation for 10 days, the appearance color of the preparation remains milky white all the time, the sedimentation ratio does not change after one day, and the content of active ingredient TylosinA decreases...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com