Antibody of TNF (Tumor Necrosis Factor) alpha and application thereof
A technology of antibodies and sequences, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve the problems of high production costs, inability to be used in clinics, and insufficient affinity of monoclonal antibodies, so as to achieve enhanced affinity, important economic value and social Significance, the effect of improving the ability to inhibit TNFα
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Materials and Method:
[0030] Recombinant human tumor necrosis factor TNFα was purchased from PeprotechAsia (Remcombinant HumanTNF-α, Cat: 300-01A).
[0031] The phage display kit was purchased from Amersham Bioscience (Recombinant phage antibody system, Cat: 27-9401-01).
[0032] See SEQ ID NO:151 for the sequence of the D2E7 heavy chain. See SEQ ID NO:152 for the sequence of the D2E7 light chain.
[0033] Linker sequence between heavy chain and light chain (SEQ ID NO: 138):
[0034] 5’-GGTGGAGGCGGTTCAGGTGGAGGCGGTTCAGGTGGAGGCGGTTCA-3’, encoded amino acid sequence (SEQ ID NO: 135): 5’-(Gly 4 Ser) 3 -3’, 15 amino acids in total.
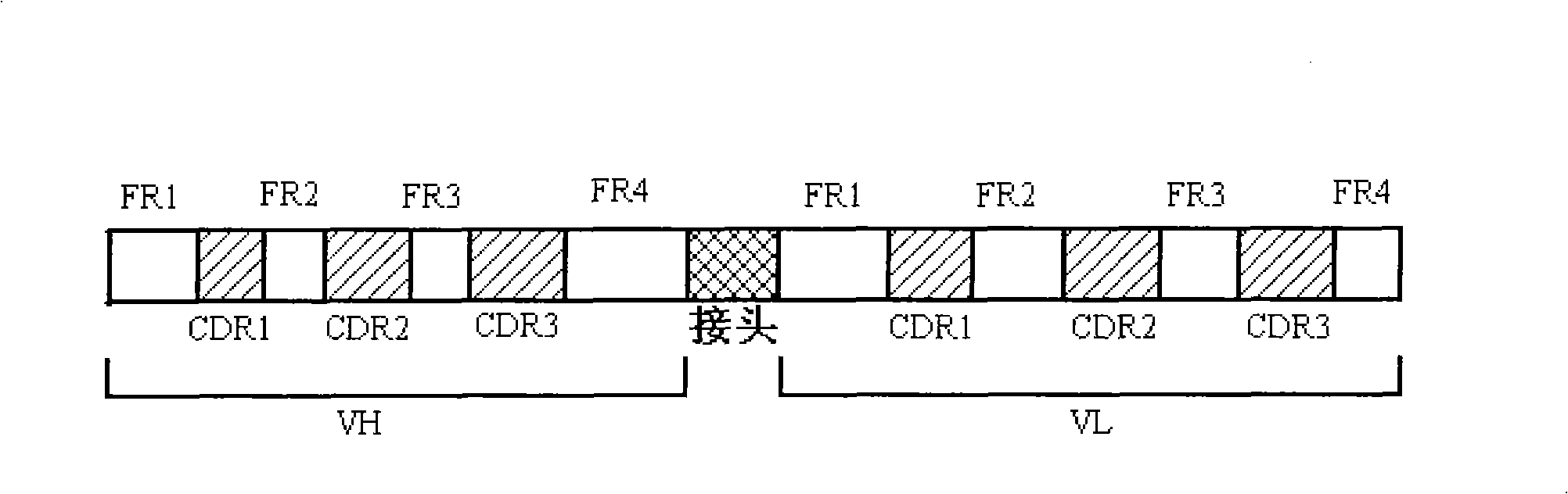
[0035] First, according to the D2E7 heavy chain, light chain and linker sequence, the complete nucleic acid sequence was artificially synthesized in the following order: (V H )FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4-linker sequence-(V L )FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4(D2E7scFv), see figure 1 .
[0036] Secondly, the primers were synthesized, and the sequence of e...
Embodiment 2
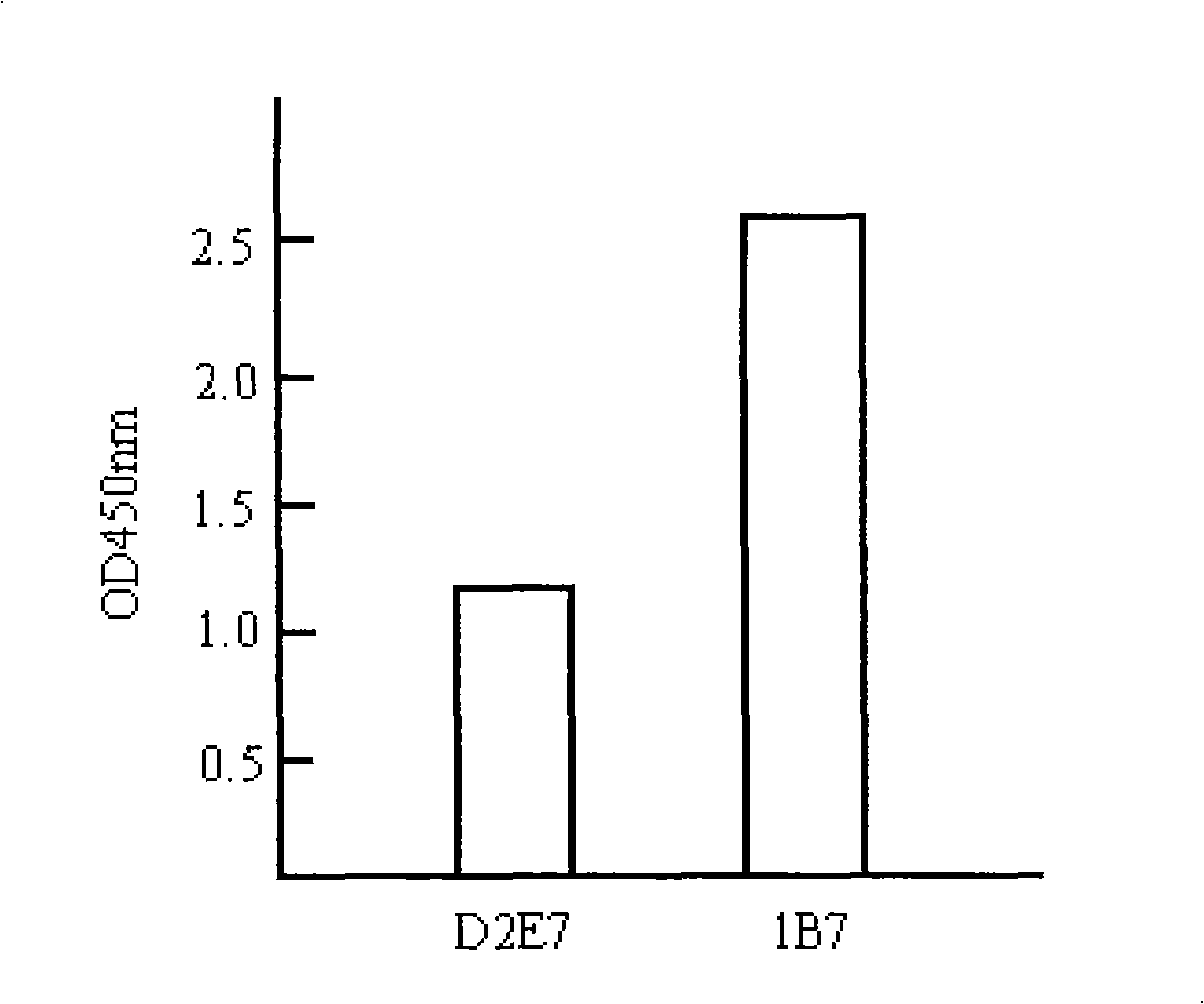
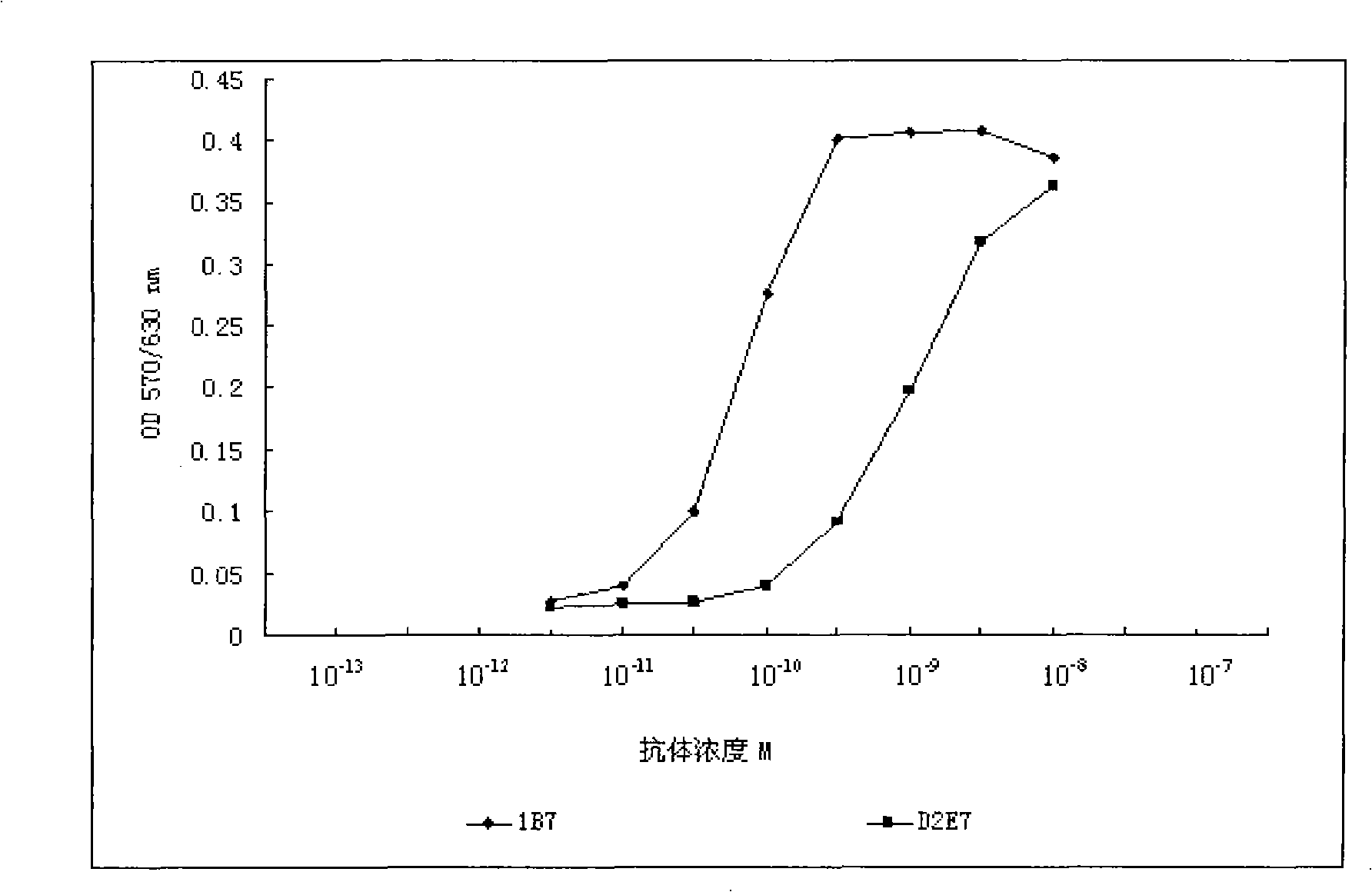
[0228] Taking 1B7 as an example, we further studied the influence of each mutant amino acid on its affinity. Compared with wild-type D2E7, clone 1B7 has one amino acid mutation in each CDR region of the heavy chain and light chain, and a total of 6 amino acids are mutated. Each mutated amino acid is back-mutated, that is, the mutated amino acid is restored. For the wild-type sequence, the results of phage Elisa showed that the amino acid recovery (His mutation to Ile) mutation in the heavy chain CDR2 region did not affect the affinity of the antibody, but the other 5 amino acid back mutations would affect the affinity of the antibody. In other words, the remaining 5 amino acid mutations can all increase the affinity of D2E7.
[0229] The mutation method and the phage Elisa method are as described above.
Embodiment 3
[0230] Example 3: Expression and purification of full-length antibodies
[0231] Materials and Method:
[0232] Chinese hamster ovary cell CHO was purchased from ATCC, recombinant protein A rProtein A was purchased from GE Healthcare Bio-Sciences AB (rProtein A Sepharose Fast Flow, Cat: 17-1279-03), and protein G (Protein G) was purchased from GE Healthcare Bio-Sciences AB (rProtein A Sepharose 4FastFlow, Cat: 17-0618-06).
[0233] Full-length antibodies, including the variable and constant regions of the heavy chain (human gamma1, gamma2, gamma3, and gamma4 regions), and the variable and constant regions of the light chain (human kappa or lambda regions), usually need to be breast-feeding Animal cell expression has therapeutic activity. Commonly used mammalian cells include CHO, NS0, COS, SP2, 293 and other cells. The heavy chain and light chain of an antibody can be expressed separately on different vectors or on the same vector. Carriers usually require screening marker genes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com