Nitrogen-containing heterocyclic thienopyridine compounds and preparation method and application thereof
A compound, pyridine technology, applied in the field of medicine, can solve the problems of low specificity, toxic side effects, poor selectivity, etc.
Inactive Publication Date: 2010-09-29
TIANJIN INSTITUTE OF PHARMA RESEARCH
View PDF1 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Traditional chemotherapeutic drugs have obvious clinical therapeutic effects, but their disadvantages are: low specificity and poor selectivity, leading to obvious toxic and side effects, and prone to serious tumor multi-drug resistance, which limits clinical application. Looking for safe and effective anti-tumor drugs Drugs have always been the pursuit of the pharmaceutical industry
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
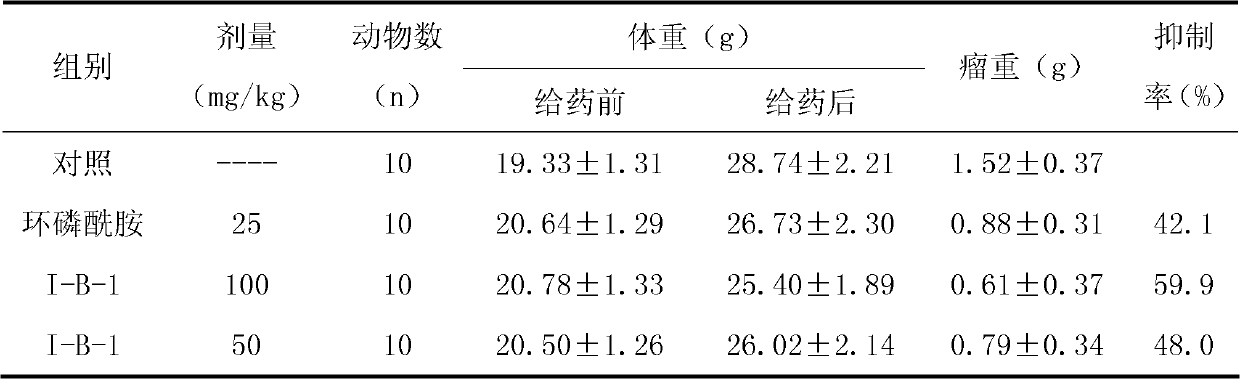
The invention belongs to the technical field of drugs for resisting malignant tumor and provides nitrogen-containing heterocyclic thienopyridine compounds with the structure shown as a general formula I and salts thereof, wherein R1 is hydrogen, and C1-C4 are alkanoyloxy substituted by alkyl; and R2 is nitrogen-containing six-membered heterocycle, i.e. benzoazo six-membered heterocycle. The invention further relates to a method for preparing the compounds, and also discloses drug compositions taking the compounds or the salts thereof as the active effective components and application thereof as drugs for resisting malignant tumor.
Description
technical field The invention belongs to the technical field of medicine, more specifically, relates to a class of compounds with anti-malignant tumor effects and a preparation method thereof. Background technique Tumor is a common disease that threatens human life. According to statistics, the total number of cancer deaths in the world reaches 7 million people every year. In my country, more than 1 million people die from cancer every year, and gradually increase. It has become the first cause of death in urban population. Traditional chemotherapeutic drugs have obvious clinical therapeutic effects, but their disadvantages are: low specificity and poor selectivity, leading to obvious toxic and side effects, and prone to serious tumor multi-drug resistance, which limits clinical application. Looking for safe and effective anti-tumor drugs Drugs have always been the pursuit of the pharmaceutical industry. Contents of the invention One object of the present invention is to...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D495/04A61K31/4709A61K31/444A61P35/00A61P35/02
Inventor 刘登科刘颖刘默刘冰妮周云松祁浩飞侯佳佳王平保
Owner TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com