Alkyl modified nucleic acid and modification method and application thereof
A nucleic acid and alkyl technology, applied in the field of nucleic acids, can solve the problems of limited improvement of siRNA stability, and achieve the effects of simple and easy reaction, chemical stability and blood retention time improvement, good lipophilicity and membrane permeability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Tumor cell telomerase RNA has 11 nucleotides, which is used as a template to design the antisense oligonucleotide sequence of this nucleotide:
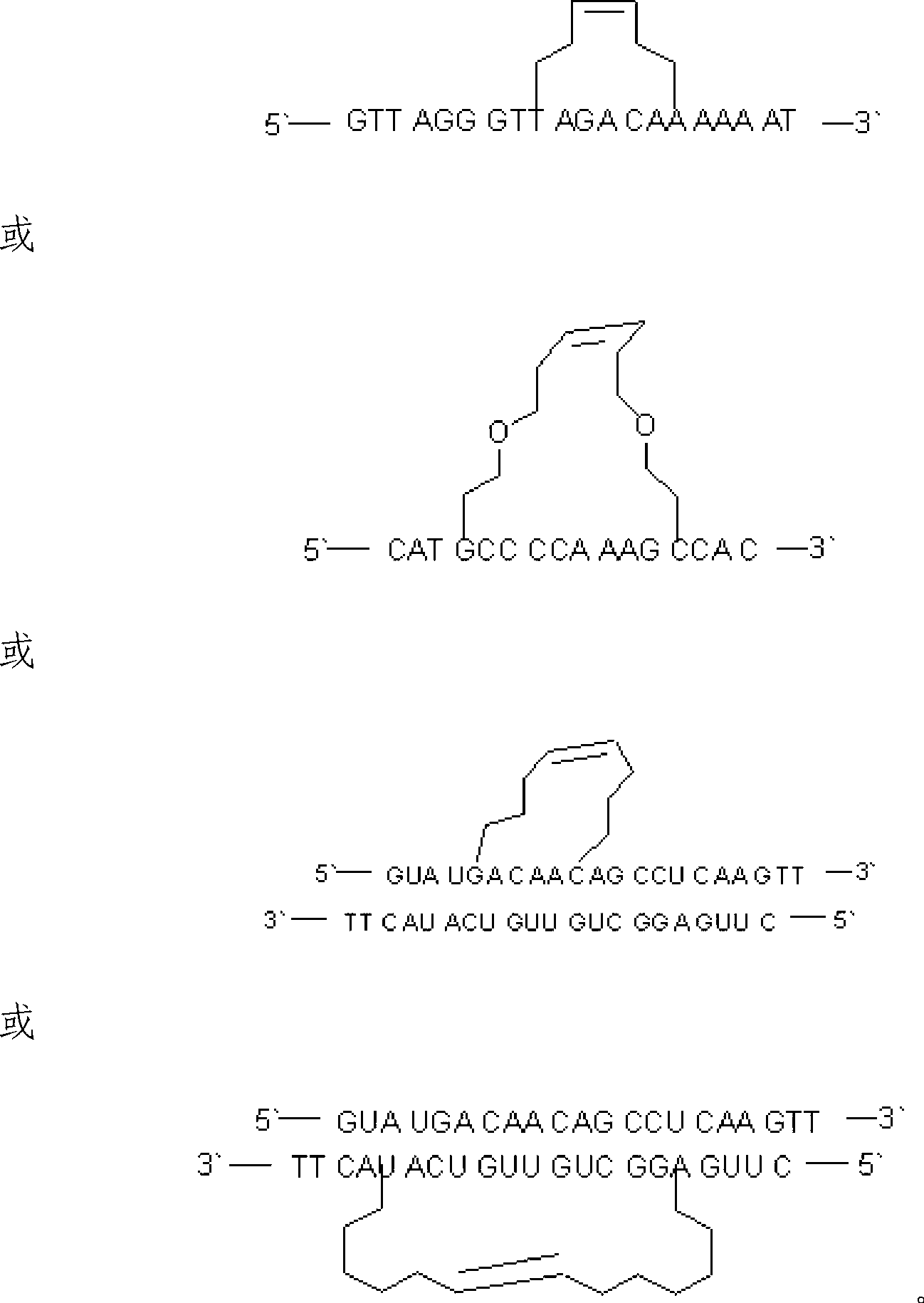
[0066] Antisense sequence: 5`-GTT AGG GTT AGA CAA AAA AT-3`
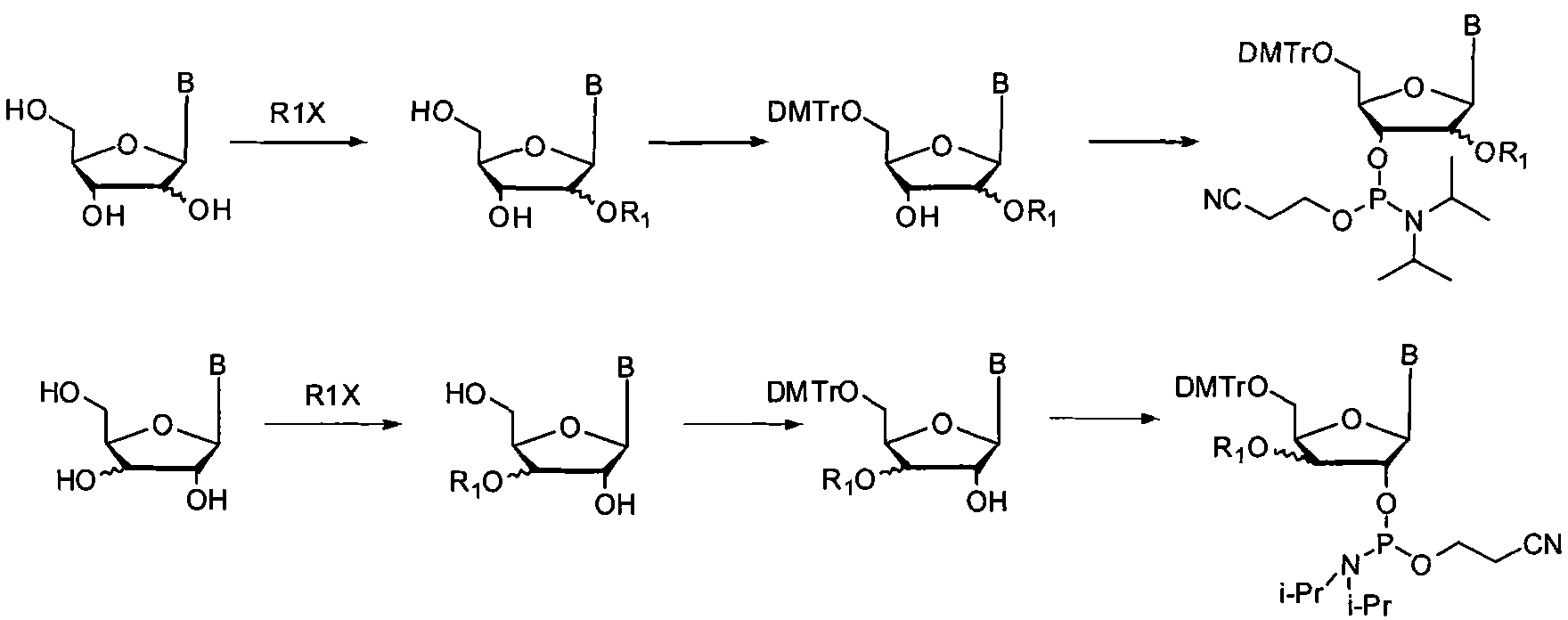
[0067] (1) preparation is selected from the nucleoside monomer shown in general formula 5 and general formula 6:
[0068]
[0069] (2) Synthesizing oligonucleotide chains by conventional solid-phase synthesis methods, and doping nucleoside monomers into the oligonucleotide chains during the synthesis process;
[0070]
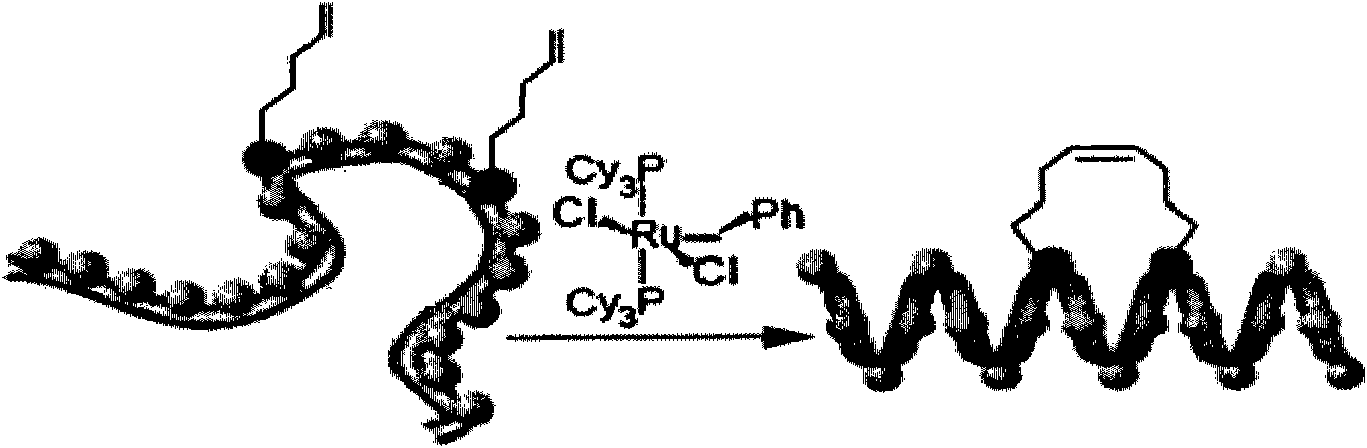
[0071] (3) Olefin ring-closing metathesis reaction occurs under the action of Grubbs catalyst, and side chain olefins are coupled into alkane chains.
[0072]
Embodiment 2
[0074] According to the gene U5 sample sequence of hepatitis B, design the antisense oligonucleotide sequence of this section nucleotide:
[0075] Antisense sequence: 5`-CAT GCC CCA AAG CCA C-3`
[0076] (1) preparation is selected from the nucleoside monomer shown in general formula 7 and general formula 8:
[0077]
[0078] (2) Synthesizing oligonucleotide chains by conventional solid-phase synthesis methods, and doping nucleoside monomers into the oligonucleotide chains during the synthesis process;
[0079]
[0080] (3) Olefin ring-closing metathesis reaction occurs under the action of Grubbs catalyst, and side chain olefins are coupled into alkane chains.
[0081]
Embodiment 3
[0082] Example 3 Application of the chemical modification method of the present invention in improving the stability of siRNA
[0083] (1) Select the target gene, determine the sequence of the sense strand and the antisense strand of the siRNA Select GAPDH (Genbank accession number is NC_000012) as the target gene, design siRNA, and its position corresponding to NC_000012 is 2700-2718bp;
[0084] Justice Chain: 5`-GUA UGA CAA CAG CCU CAA GTT-3`
[0085] Antisense strand: 5`-CUU GAG GCU GUU GUC AUA CTT-3`
[0086] (2) preparation is selected from the nucleoside monomer shown in general formula 5 and general formula 6
[0087]
[0088] (3) Solid-phase synthesis of sense and antisense strands of siRNA
[0089] Justice Chain:
[0090]
[0091] Antisense strand: 3`-TT CAU ACU GUU GUC GGA GUU C-5`
[0092] (4) Annealing and hybridization to form siRNA double strands
[0093]
[0094] (5) Olefin metathesis reaction occurs under the action of Grubbs catalyst to fix the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com