Fused bicyclic compound
A technology for fused bicyclic compounds, applied in the field of new fused bicyclic compounds
Inactive Publication Date: 2010-08-18
MITSUBISHI TANABE PHARMA CORP
View PDF5 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, there are no reports on fused bicyclic compounds such as the compounds of the present invention (1,3-benzoxazine derivatives or benzopyran derivatives) having MR modulating activity (such as MR antagonistic activity)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
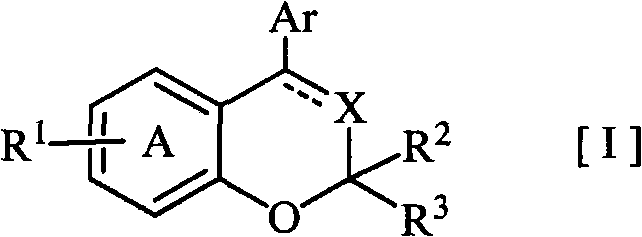
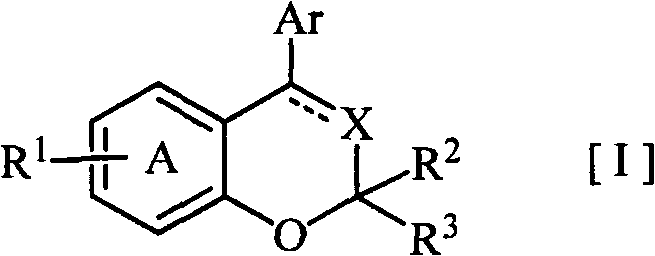
Disclosed is a novel fused bicyclic compound [I] shown below, which has affinity for a mineralcorticoid receptor (MR) and is useful as an anti-hypertensive agent or the like. Specifically disclosed is a compound represented by the formula [I] or a pharmacologically acceptable salt thereof. [I] wherein the ring A represents a benzene ring which is fused with the adjacent heterocyclic 6-membered ring and has a substituent R1, and which may have a substituent other than R1; R1 represents an alkylsulfonylamino group or the like; R2 and R3 (a) independently represent a hydrogen, an alkyl or a substituted or unsubstituted aryl, (b) together form an oxo, or (c) together with the adjacent carbon atom, form a cycloalkyl; X represents =N-, =C(R4)- or -CH(R4)-; R4 represents (a) a hydrogen, (b) a cyano, (c) a halogen, (d) an alkyl, (e) an alkenyl, (f) a cycloalkyl, (g) an alkanoyl, (h) a carbamoyl or (i) a cycloalkenyl; Ar represents a substituted or unsubstituted aromatic cyclic group; and a dotted line means the presence or absence of a double bond.
Description
technical field The present invention relates to novel fused bicyclic compounds having an affinity for the mineralocorticoid receptor (MR) which are useful in the treatment and / or prophylaxis of diseases or clinical conditions associated with said receptor. Background technique Physiologically active hydrophobic substances having a low molecular weight such as steroid hormones demonstrate their actions via the respective nuclear receptors as their ligands. A group of steroid hormone nuclear receptors forms an inherited superfamily and controls, ie, activates or represses, target gene expression at the transcriptional level by functioning as ligand-dependent transcription factors. Steroid hormone receptors include mineralocorticoid receptor (MR), glucocorticoid receptor (GR), androgen receptor (AR), estrogen receptor (ER) and progesterone receptor (PR). Steroid hormones such as mineralocorticoids (aldosterone) or glucocorticoids (cortisone, etc.), which are ligands for rece...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D265/16A61P9/12A61P43/00C07D311/58C07D413/04A61P9/04A61K31/352A61K31/353A61K31/536A61P9/00
CPCC07D413/04C07D311/96C07D405/14C07D265/16C07D311/58C07D311/20A61P5/46A61P9/00A61P9/04A61P9/06A61P9/12A61P43/00
Inventor 高桥阳一淡井信将赤塚英则川口隆行饭岛彻
Owner MITSUBISHI TANABE PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com