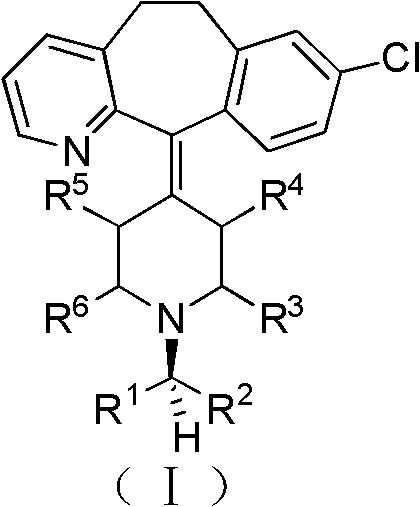
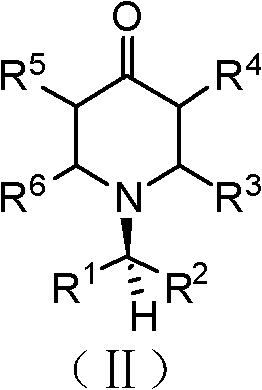
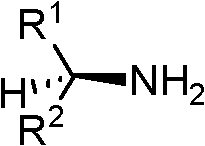
Chiral tricyclic compounds with anti-histamine activity, preparation method and application
A compound, antihistamine technology, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problem of unreported synthesis of chiral derivatives, and achieve the effect of strong antihistamine activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Add methyl acrylate (11.33g, 0.13mol) to a dry 100mL four-neck flask, slowly add (R)-2-amino-1-propanol (3.76g, 0.05mol) dropwise under stirring at room temperature, drop After the completion, the oil bath was heated to 40°C, and the reaction was stopped after about 12 hours. The excess methyl acrylate was evaporated under reduced pressure to obtain a light yellow oil. The crude product was separated and purified by silica gel column chromatography to obtain 6.55 g of the addition product. The molar yield was 53.0%, [α] D 20 -73.6° (c 1.0, MeOH).
[0055] Its structural formula is
[0056]
[0057] 1 HNMR (CDCl 3 , 500MHz) δ: 3.67(s, 6H), 3.38(t, J=10.5Hz, 1H), 3.33(q, J=4.8Hz, 1H), 2.84~2.90(m, 3H), 2.56~2.60(m , 2H), 2.38~2.52(m, 4H), 0.88(d, J=7.0Hz, 3H);
[0058] 13 C NMR (CDCl 3 , 125MHz) δ: 173.25, 63.19, 57.08, 51.82, 44.91, 33.80, 9.29.
[0059] (2) Add 200mL of toluene to a 500mL four-neck flask with mechanical stirring, heat up to reflux, then ...
Embodiment 2
[0070] (1) Add methyl acrylate (8.60g, 0.1mol) to a dry 100mL four-neck flask, slowly add (S)-2-amino-1-propanol (3.76g, 0.05mol) dropwise under stirring at room temperature, drop After the completion, the oil bath was heated to 80°C, and the reaction was stopped after about 14 hours. The excess methyl acrylate was evaporated under reduced pressure to obtain a light yellow oil. The crude product was separated and purified by silica gel column chromatography to obtain 7.73 g of the addition product, and the molar yield was 62.5%, [α] D 20 +69.5° (c 1.0, MeOH).
[0071] Its structural formula is
[0072]
[0073] 1 H NMR (CDCl 3 , 500MHz) δ: 3.67(s, 6H), 3.37(t, J=10.5Hz, 1H), 3.33(q, J=5.3Hz, 1H), 2.84~2.90(m, 3H), 2.56~2.60(m , 2H), 2.46~2.52(m, 2H), 2.38~2.43(m, 2H), 0.88(d, J=6.5Hz, 3H);
[0074] 13 C NMR (CDCl 3 , 125MHz) δ: 173.06, 62.96, 56.86, 51.63, 44.69, 33.57, 9.07.
[0075] (2) Add 230mL tetrahydrofuran in a 500mL four-necked flask with mechanical stirri...
Embodiment 3
[0086] (1) Add methyl acrylate (2.15g, 0.025mol) to a dry 100mL four-neck flask, slowly add (R)-2-amino-1-butanol (0.89g, 0.01mol) dropwise under stirring at room temperature, and heat up to 35°C, stop the reaction for about 20 hours, distill off methanol and excess methyl acrylate to obtain a crude product, and separate the residue by silica gel column chromatography to obtain 1.29 g of an addition product with a molar yield of 49.7%, [α] D 20 +20.2° (c 1.0, MeOH).
[0087] Its structural formula is
[0088]
[0089] 1 H NMR (CDCl 3 , 300MHz) δ: 3.74(s, 6H), 3.59(q, J=5.7Hz, 1H), 3.38(t, J=10.4Hz, 1H), 2.92~2.97(m, 2H), 2.71~2.75(m , 3H), 2.50~2.61(m, 4H), 1.57~1.68(m, 1H), 1.18~1.34(m, 1H), 0.97(t, J=7.5Hz, 3H);
[0090] 13 C NMR (CDCl 3 , 75MHz) δ: 172.99, 63.99, 60.80, 51.60, 45.35, 33.89, 18.39, 11.90.
[0091] (2) Add 220mL of dioxane into a 500mL four-neck flask with mechanical stirring, heat up to reflux, then add potassium tert-butoxide (9.76g, 0.087mol) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com