Pharmaceutical intermediate, preparation method thereof and method for preparing iloperidone by pharmaceutical intermediate
A technology for medicinal salts and compounds, which is applied in the field of preparing iloperidone, iloperidone intermediates and their preparation, can solve the problems of being unsuitable for large-scale production, low coupling reaction yield, difficult to remove impurities, and the like, Achieve the effect of suitable for large-scale industrial production, convenient post-processing and outstanding yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
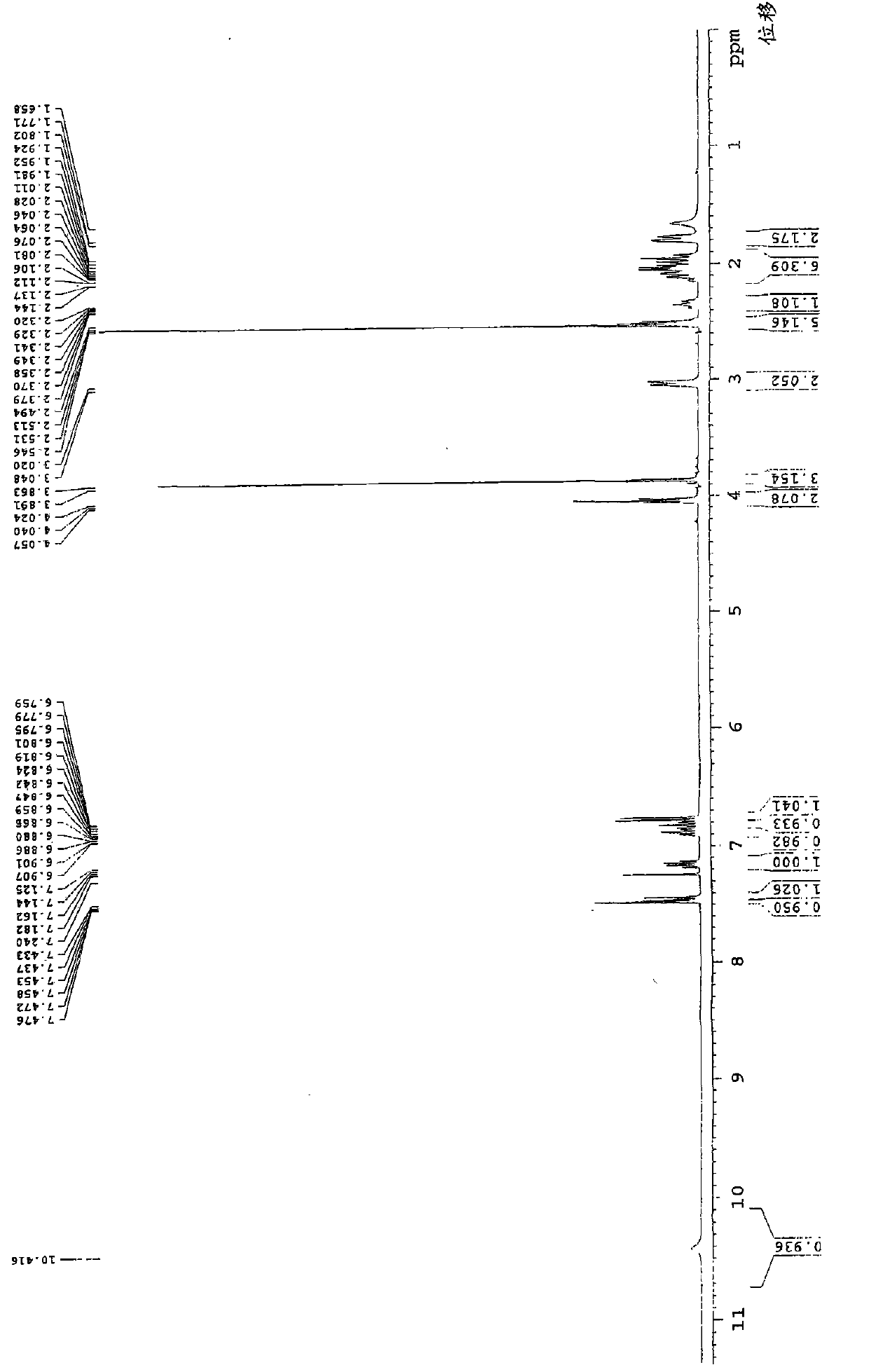
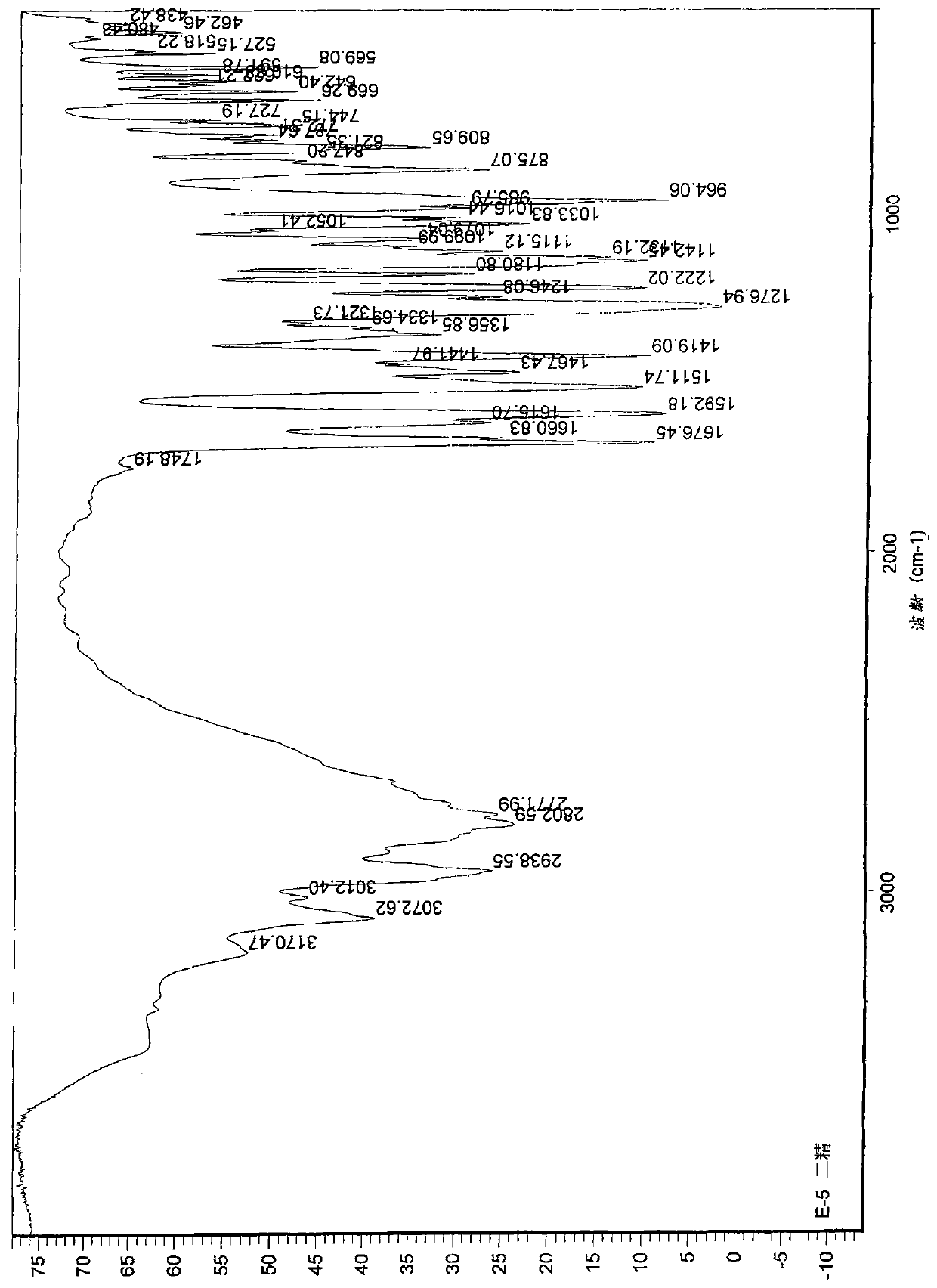
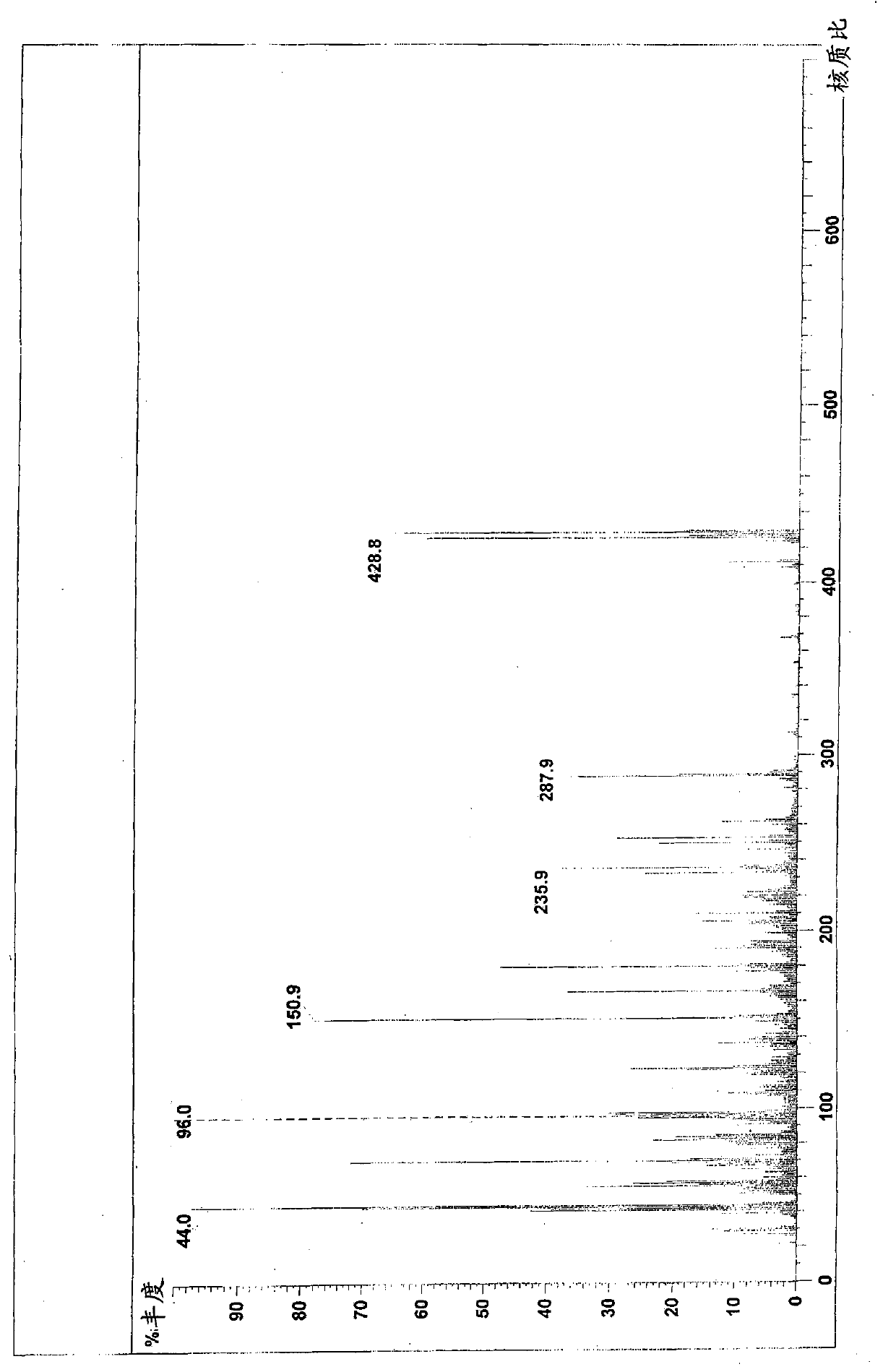
[0043] 4-[3-[4-(2,4-difluorophenyl)-(hydroxyimino)methyl]-1-piperidinyl]propoxy]-3-methoxyacetophenone (formula 1 Compound) preparation:
[0044] a) raw material compound: 2, the preparation of 4-difluorophenyl (4-piperidinyl) ketone oxime (compound of formula 2):
[0045] Mix 30g of 2,4-difluorobenzene-(4-piperidinyl)methanone hydrochloride (purchased by Beijing Gaobo Pharmaceutical Chemical Technology Development Co., Ltd.), 30g of hydroxylamine hydrochloride and 300ml of absolute ethanol, and heat to 80°C for reflux , stirring, the rotating speed is 120~130 rpm, and the reaction is 5 hours. Cool to room temperature, filter, mix the filter cake with 90ml of water, adjust the pH value to ≥11 with 30% potassium hydroxide aqueous solution, filter, and vacuum dry below 60°C to obtain 25g of white solid. The obtained white solid was tested by a YRT-3 melting point apparatus, and the melting point of the white solid was 214-218° C. after testing. Therefore, it can be seen that ...
Embodiment 2
[0051] 4-[3-[4-(2,4-difluorophenyl)-(hydroxyimino)methyl]-1-piperidinyl]propoxy]-3-methoxyacetophenone (formula 1 The preparation of the shown compound):
[0052] 4.8g 2,4-difluorophenyl (4-piperidinyl) ketone oxime, 5g 4-chloropropoxy-3-methoxyacetophenone, 3.5g potassium carbonate, 0.1g sodium iodide and 60ml of N,N-dimethylformamide was mixed, heated and stirred, and reacted at 80°C for 8 hours. Cool to room temperature, add 200ml of water and 80ml of dichloromethane for liquid separation, wherein the water layer in the reaction system is extracted twice with dichloromethane, 50ml each time, the organic layer is combined, washed with water, and dried with anhydrous magnesium sulfate , and concentrated to dryness to obtain 7.7 g of the product with a yield of 86.5%. The product is tested by a YRT-3 melting point apparatus, and the melting point of the product is 138-142° C. after testing. Therefore, it can be seen that the resulting product is a compound 4-[3-[4-(2,4-difl...
Embodiment 3
[0054] 4-[3-[4-(2,4-difluorophenyl)-(hydroxyimino)methyl]-1-piperidinyl]propoxy]-3-methoxyacetophenone (formula 1 Shown compound) the preparation of hydrochloride:
[0055] With 6g 4-[3-[4-(2,4-difluorophenyl)-(hydroxyimino)methyl]-1-piperidinyl]propoxy]-3-methoxyacetophenone and Mix and stir with 24ml of ethanol, the stirring speed is 120-130 rpm, add hydrochloric acid ethanol solution dropwise to the reaction system, so that the pH of the reaction system is ≤2, after the white solid is precipitated, filter and vacuum dry below 60°C, 6 g of product were obtained in a yield of 93.7%. The product is tested by a YRT-3 melting point apparatus, and the melting point of the product obtained through testing is 190-193°C. Therefore, the obtained product is 4-[3-[4-(2,4-difluorophenyl)-(hydroxyimino)methyl]-1-piperidinyl]propoxy]-3-methoxy Acetophenone (compound shown in formula 1) hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com