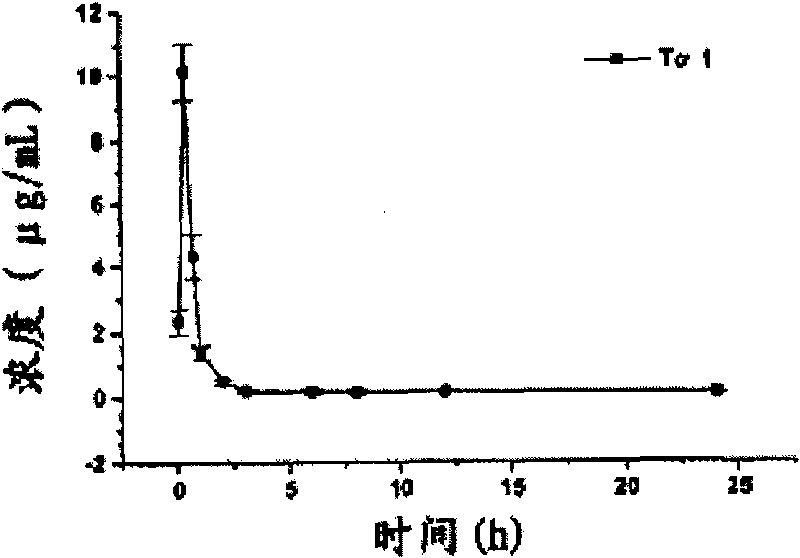
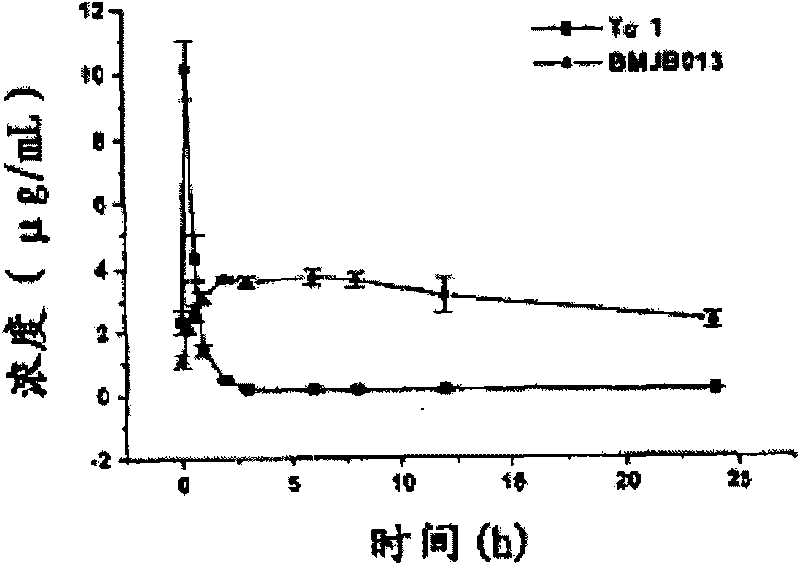
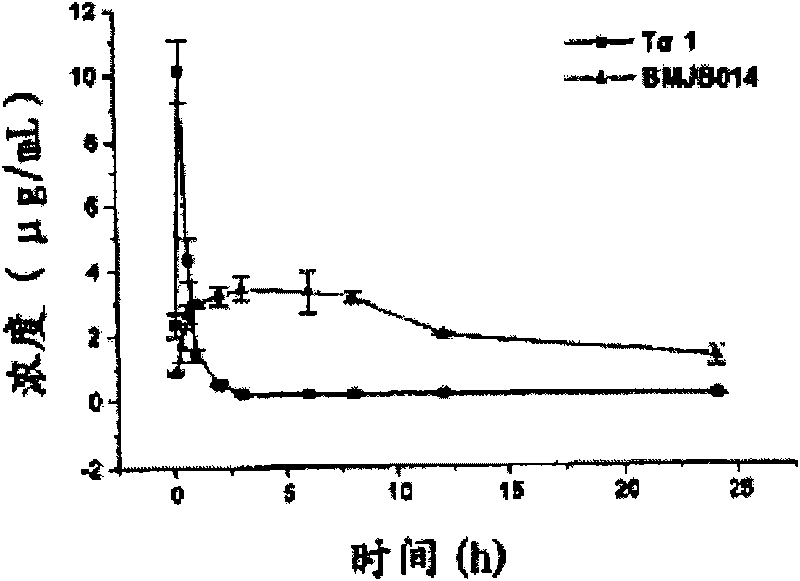
Long-acting thymosin alpha1-polyethylene glycol modifiers
A modification and compound technology, which is applied in the field of long-acting thymosin α1 pegylated modification, can solve the problems of frequent injections, large dosage, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1: Ac-[Cys 5 (mPEG 5000 -MAL)] Tα1 (BTJB005)
[0171] 1.1Ac-[Cys 5 ] Synthesis of Tα1:
[0172] Synthesized by solid-phase method [Cys 5 ]Tα1: Wang resin 100mg is the solid phase carrier, Fmoc-AA-OH (1-10 times the amount) is the raw material, DCC (1-10 times the amount), HOBt (1-10 times the amount) is the condensation agent, divided by Cys 5 Replace Val 5 In addition, the remaining amino acid residues are synthesized according to the Tα1 sequence to obtain Ac-[Cys 5 ] Fully protected peptide resin of Tα1. After drying, TFA was cracked, reacted at room temperature for 20-200 minutes, filtered off the resin, added diethyl ether to precipitate a white solid, dissolved in water, and lyophilized to obtain 161 mg of a white solid. RP-HPLC purification, ESI-MS identification, [M] 2+ Peak: 1551.0 (theoretical: 3098).
[0173] 1.2Ac-[Cys 5 (mPEG 5000 Synthesis of -MAL)]Tα1
[0174] Purified Ac-[Cys 5 ] Tα1 is dissolved in water, adjust the pH range of 5-10...
Embodiment 2
[0176] Example 2: Ac-[Cys 8 (mPEG 5000 -MAL)] Tα1 (BMJBO08)
[0177] Synthesize Ac-[Cys according to embodiment 1.1 method 8 ] Tα1 to obtain 116 mg of crude peptide. RP-HPLC purification, ESI-MS identification, [M] 2+ Peak: 1543.0 (theoretical: 3083).
[0178] Synthesize Ac-[Cys according to the method of embodiment 1.2 8 (mPEG 5000 -MAL)]Tα1, the product was separated by HPLC and lyophilized to obtain 16.9 mg of white solid, with a yield of 37.1%.
[0179] Ac-[Cys 8 (mPEG 5000 -MAL)]Tα1 was identified by MALDI-TOF-MS, and there were a series of peaks around 8247, and the molecular weight difference between the two adjacent peaks was about 44, which had the typical structural characteristics of polyethylene glycol.
Embodiment 3
[0180] Example 3: Ac-[Cys 11 (mPEG 5000 -MAL)] Tα1 (BMJB011)
[0181] Synthesize Ac-[Cys according to embodiment 1.1 method 11 ] Tα1 to obtain 143 mg of crude peptide. RP-HPLC purification, ESI-MS identification, [M] 3+ Peak: 1034.9 (theoretical: 3098).
[0182] Synthesize Ac-[Cys according to the method of embodiment 1.2 11 (mPEG 5000 -MAL)]Tα1, the product was separated by HPLC and lyophilized to obtain 21.4 mg of white solid, with a yield of 28.9%.
[0183] Ac-[Cys 11 (mPEG 5000-MAL)]Tα1 was identified by MALDI-TOF-MS, and there were a series of peaks around 8282, and the molecular weight difference between the two adjacent peaks was about 44, which had the typical structural characteristics of polyethylene glycol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com