Two crystal form materials of salvianolic acid A, preparation method as well as medicine compound and application thereof
A technology of salvianolic acid and crystal form, applied in directions such as drug combination, preparation of carboxylate, preparation of organic compounds, etc., can solve problems such as no reports on the preparation method of solid crystal material.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
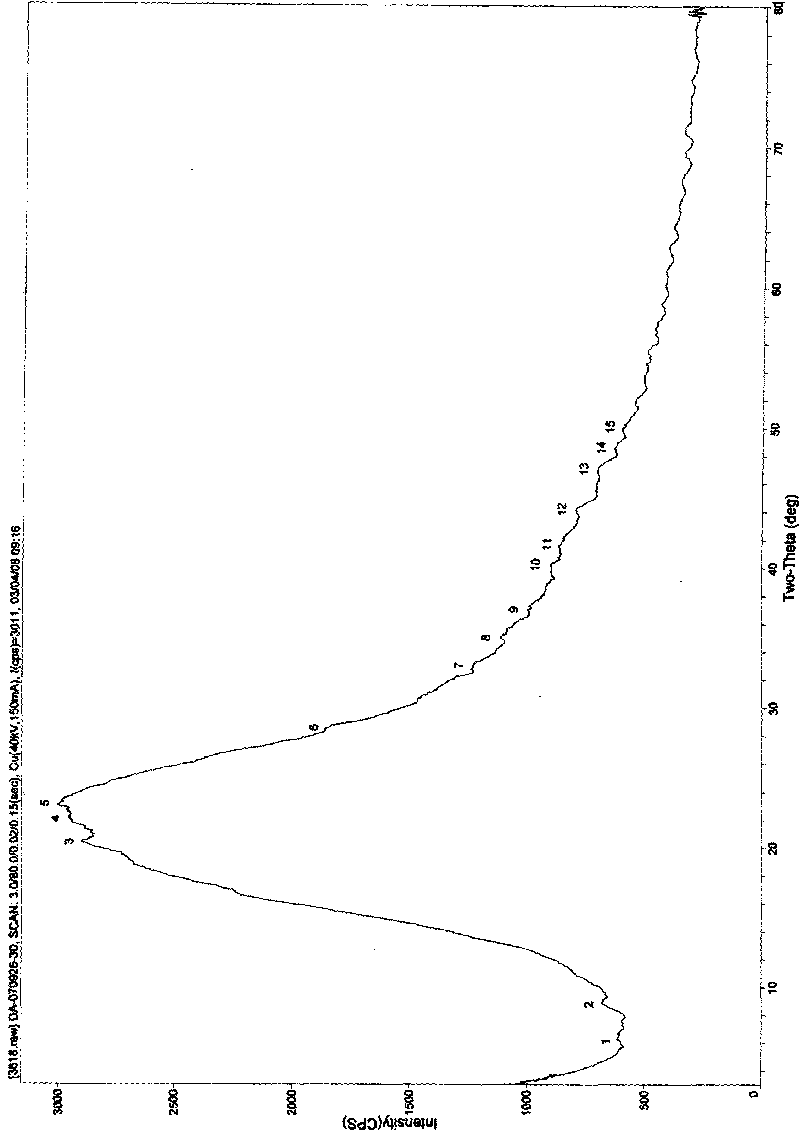
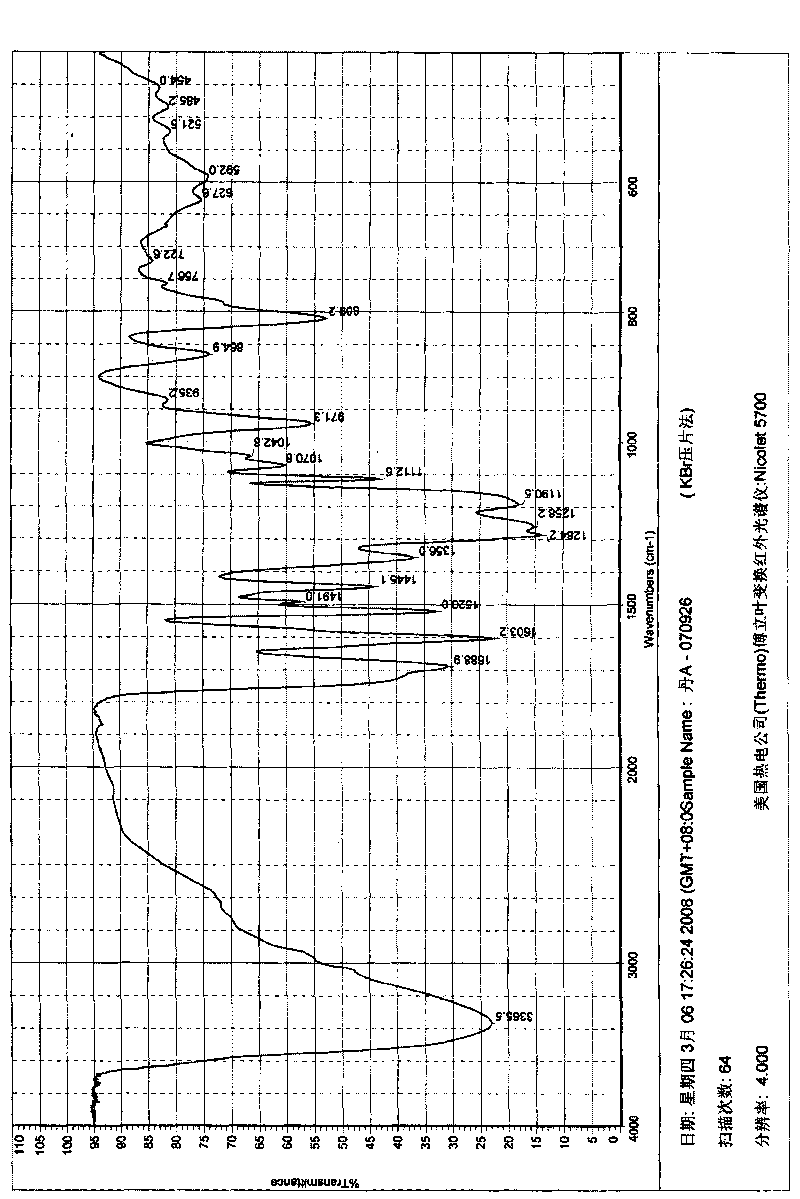
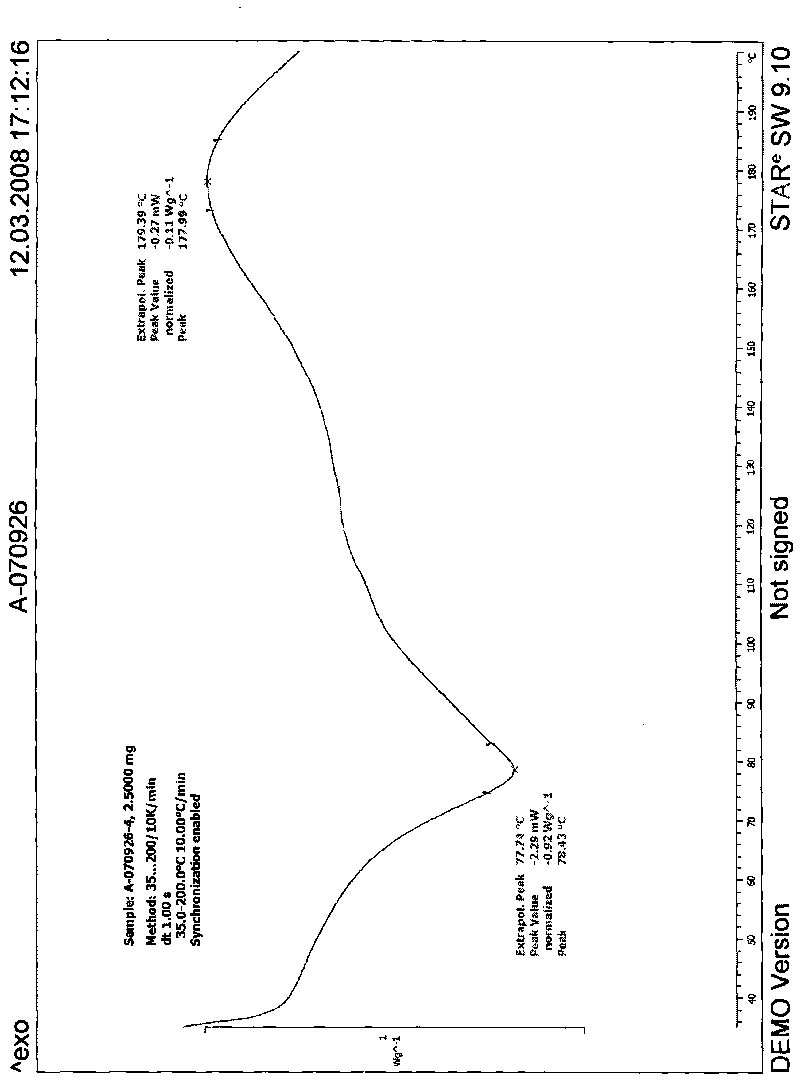
Image
Examples
Embodiment 1
[0086] Preparation method 1 of the α crystal form solid sample of salvianolic acid A:
[0087] The preparation method of the α crystal form solid sample of salvianolic acid A is characterized in that the salvianolic acid A sample is completely dissolved at 15-25°C at normal temperature using acetone or ethanol solvent, and the temperature is controlled at 45°C by vacuum filtration or spinning. Evaporation method The solvent in the sample is quickly suction filtered or evaporated to dryness, and the sample is then dried to finally prepare the α-crystal solid sample of salvianolic acid A.
[0088] Preparation method 2 of the α crystal form solid sample of salvianolic acid A:
[0089] The preparation method of the α-crystal form solid sample of salvianolic acid A is characterized in that the mixed solution is prepared by using n-propanol and water at a ratio of 2:1, and then the salvianolic acid A sample is completely dissolved and controlled under normal temperature of 15-25°C. ...
Embodiment 2
[0095] Preparation method 1 of the β crystal form solid sample of salvianolic acid A:
[0096] The preparation method of the β-crystal form solid sample of salvianolic acid A is characterized in that the α-crystal form solid sample of salvianolic acid A is placed in a thermostat at 105°C and heated for 60 minutes to obtain the β-crystal form of salvianolic acid A. type solid samples.
Embodiment 3
[0098] Pharmacodynamic characteristics of oral administration of crystalline components of salvianolic acid A:
[0099] Experimental animals: 120 SD male rats, weighing 180-200 g, purchased from Beijing Weitong Lihua Experimental Technology Co., Ltd., obtained type 2 diabetes model rats through modeling experiments, and detected TG, TC, LDL- Blood lipid levels such as C and NEFA and blood biochemical indicators such as SOD and MDA prove that the model of type 2 diabetic rats was successfully established.
[0100] Experimental method: For rats with type 2 diabetes, after fasting for 12 hours, each 3 mg / kg of salvianolic acid crystal form solid drug was administered by intragastric administration, once a day, for 6 consecutive weeks. Before administration and 3 days, 1 week, 2 weeks, and 6 weeks after administration, blood was taken from the eye sockets, and the body weight of rats was measured before administration and 3 days, 1 week, 2 weeks, and 3 weeks after administration. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com