Novel method and compositions
A vaccine composition, mammalian technology, applied in the field of novel and composition, which can solve the problem of impossibility, impossibility, cumbersomeness of boosting viral vector components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
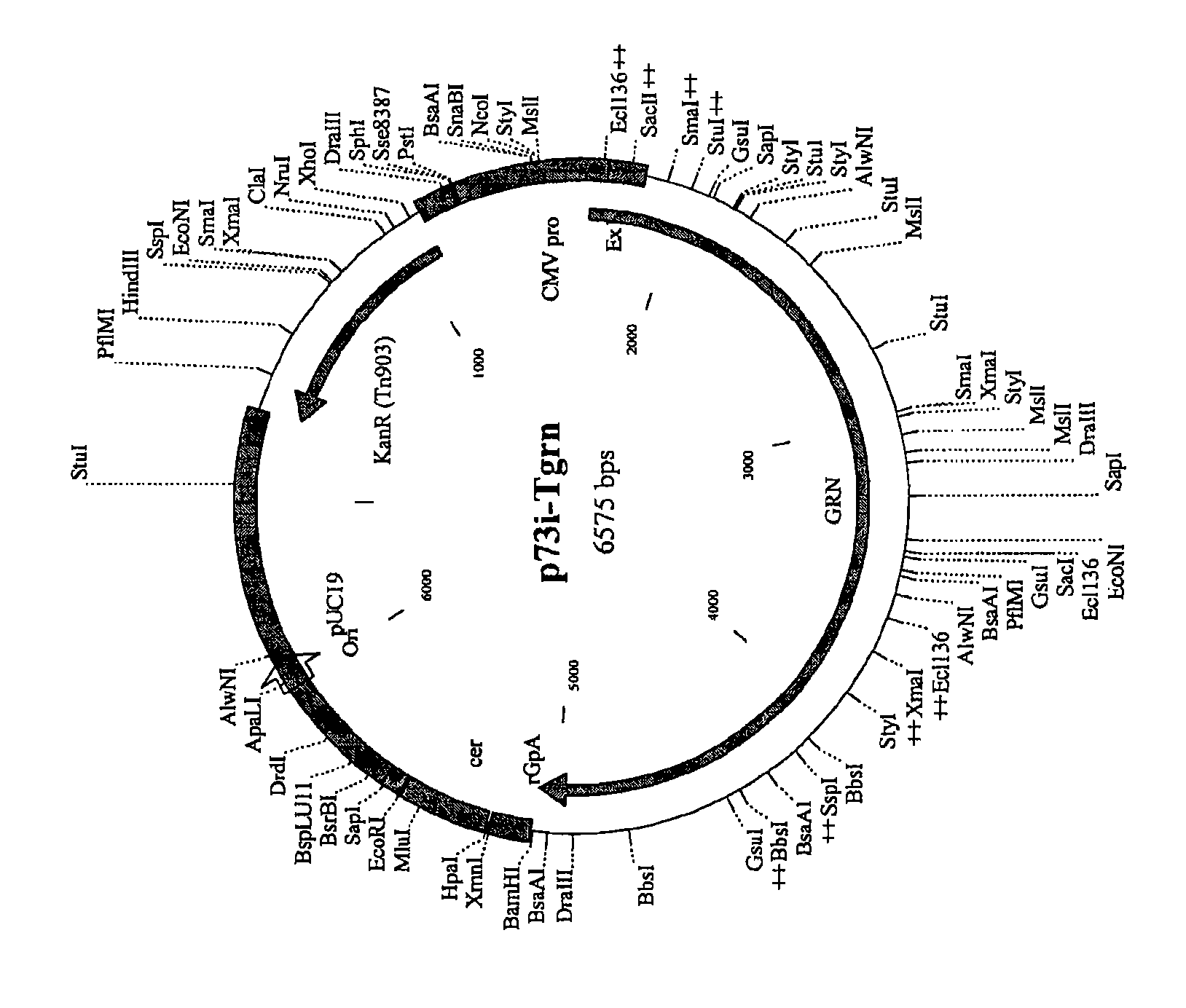
[0297] Immunization with adenovirus component (Pan7GRN) and protein component (F4 / adjuvant B) alone or with adenovirus Immunogenicity studies in mice immunized with formulations co-formulated with viral and protein components
[0298] The mouse strain used was CB6F1 with 3 mice per time point. For immunizations with F4 / Adjuvant B (P), 1 / 10 the human dose, ie, 9 μg F4 protein / 50 μl Adjuvant B, was injected. For immunizations with Pan7GRN (A) 10×10 8 Virions / 50 μl saline (0.9% NaCl in water for injection). Pan7GRN chimpanzee adenovirus carries genes encoding Gag(G), RT(R) and Nef(N).
[0299] The vaccination schedule is as follows:
[0300] group
Day 0
Day 21
Day 42
Day 63
1
-
-
F4 / adj B
F4 / adj B
2
-
-
Pan7GRN
Pan7GRN
3
F4 / adj B
F4 / adj B
Pan7GRN
Pan7GRN
4
Pan7GRN
Pan7GRN
F4 / adj B
F4 / adj B
5
-
-
-
F4...
Embodiment 2
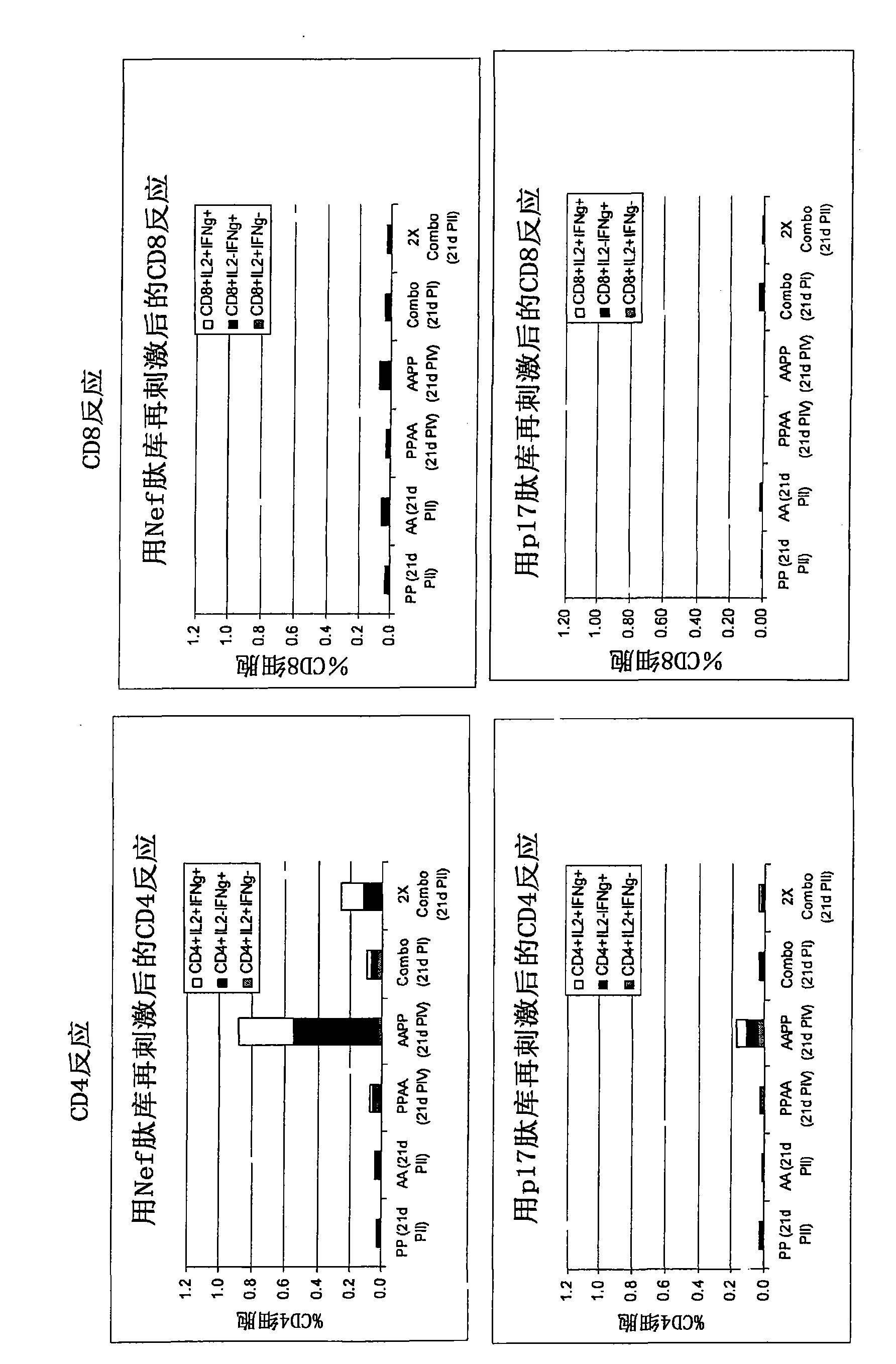
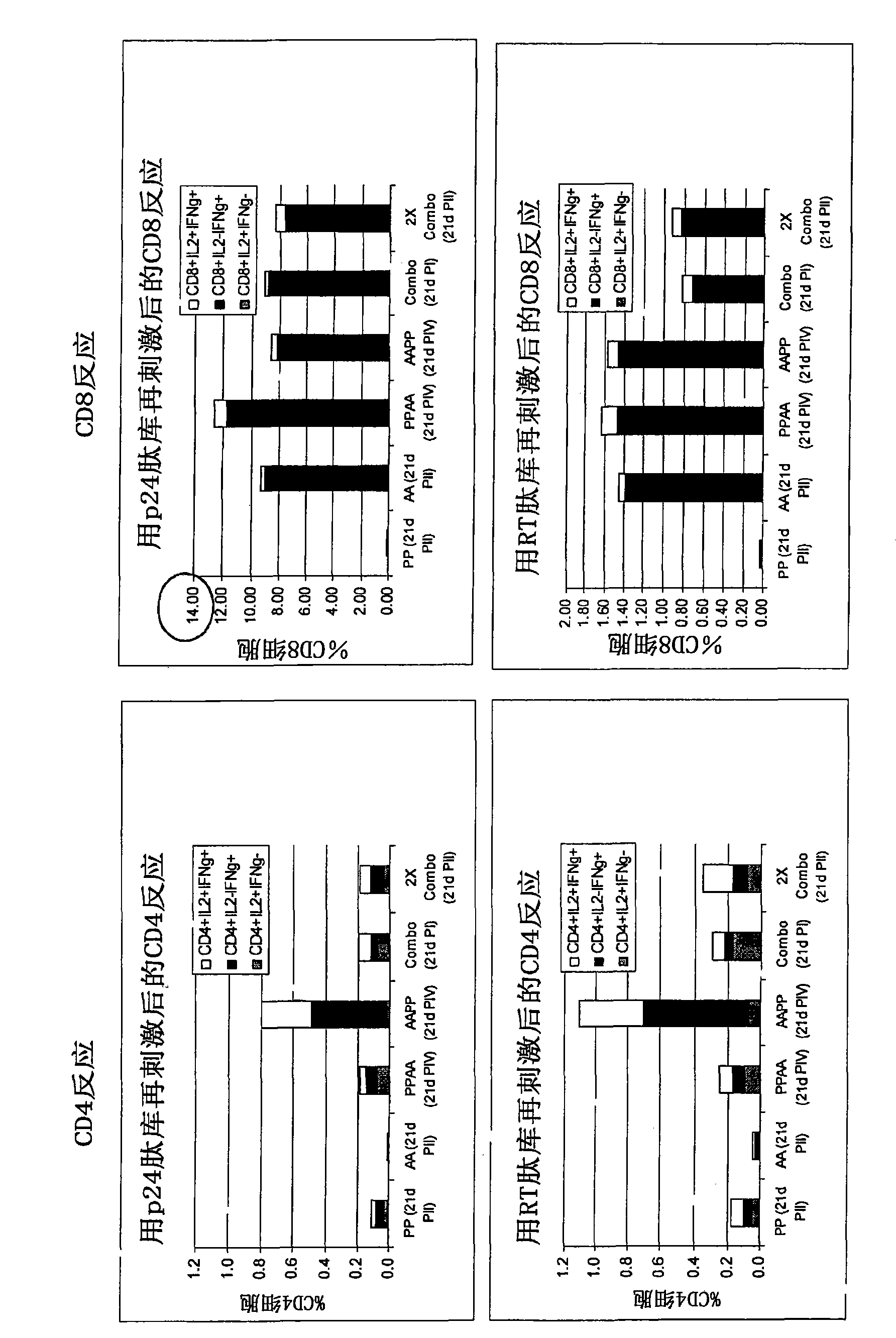
[0335] After immunization with Pan7GRN adenovirus co-formulated with F4 protein / adjuvant B Immunogenicity studies in mice
[0336] The mouse strain used was CB6F1, with 9 mice per group. F4 protein (injected 1 / 10 human dose, i.e., 9 μg) with 10×10 in 50 μL Adjuvant B or its dilution (1 / 2, 1 / 41 / 10) 8 Mice were immunized once with a co-formulation of each Pan7GRN virion. CD4 and CD8 cell responses against Nef, p17, p24 or RT peptide pools (3 pools of 3 spleens per group) were determined 21 days after immunization.
[0337] Do the following readout:
[0338] cellular response ( Figure 9 ):
[0339] - Measured by restimulation of splenocytes overnight with p24, RT, Nef or p17 peptide pools followed by surface and intracellular cytokine staining followed by flow cytometry analysis. Splenocytes were collected for analysis (3 pools of 3 spleens per group).
[0340] result:
[0341] Figure 9 The results shown in represent the cellular responses observed after restimulati...
Embodiment 3
[0352] After immunization with Pan7GRN or sequential immunization with F4 / adjuvant B or adenovirus and protein component one Immunogenicity studies in New Zealand white rabbits immunized with co-formulated preparations
[0353] For immunizations with F4 / Adjuvant B, a human dose, ie, 90 μg F4 protein / 500 μL Adjuvant B, was injected. For immunizations with Pan7GRN, use 10 × 10 10 or 10×10 12 Viral particles / 500 μL saline. For immunizations with formulations co-formulated with adenovirus and protein components, use 90 μg F4 protein per 500 μL Adjuvant B, 10×10 11 Pan7GRN virions.
[0354] The vaccination schedule is as follows:
[0355] Group
do not
Day 0
Day 14
Day 126
1
F4 / adj B
F4 / adj B
F4 / adj B
2
Pan7GRN 10^10
Pan7GRN 10^10
3
Pan7GRN 10^12
Pan7GRN 10^12
4
F4 / adj B / Pan7GRN
10^11
F4 / adj B / Pan7GRN
10^11
F4 / adj B / Pan7GRN
10^11
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com