Preparation method of cyclodextrin derivatives capable of grafting silk fabric
A technology of cyclodextrin and derivatives, applied in the direction of animal fiber, textile and papermaking, fiber processing, etc., can solve the problems of low grafting rate and silk damage, and achieve the effect of good grafting effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
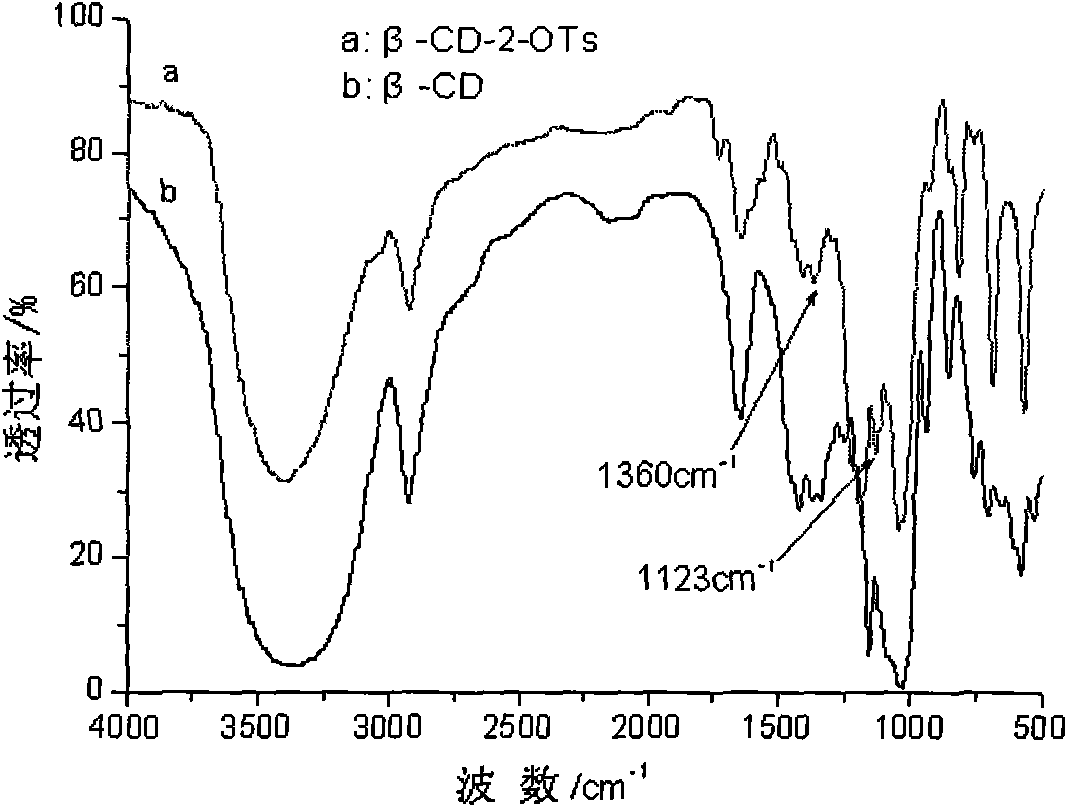
[0032] Synthesis of 2-tosylated-β-cyclodextrin (β-CD-2-OTs): add 8g β-CD (approx. 7.06mmol) and 300ml of 0.15mol / L NaOH aqueous solution, fully stirred to dissolve. Dissolve 6g of p-toluenesulfonyl chloride in a small amount of acetonitrile, and slowly drop it into the three-necked flask within 2 hours, and continuously replenish 1mol / L NaOH aqueous solution during the dropping process to ensure that the pH of the reaction solution is ≥ 12.5. After p-toluenesulfonyl chloride was dropped, the stirring reaction was continued for 2 hours. After the reaction is completed, neutralize to neutral with 1mol / L hydrochloric acid, filter off a small amount of unreacted p-toluenesulfonyl chloride. Vacuum rotary evaporate the filtrate to dryness, and obtain a white solid. Then desalt the solid twice with 200ml of anhydrous methanol, reflux The liquid is cooled, filtered, and evaporated to obtain the white powder product β-CD-2-OTs.
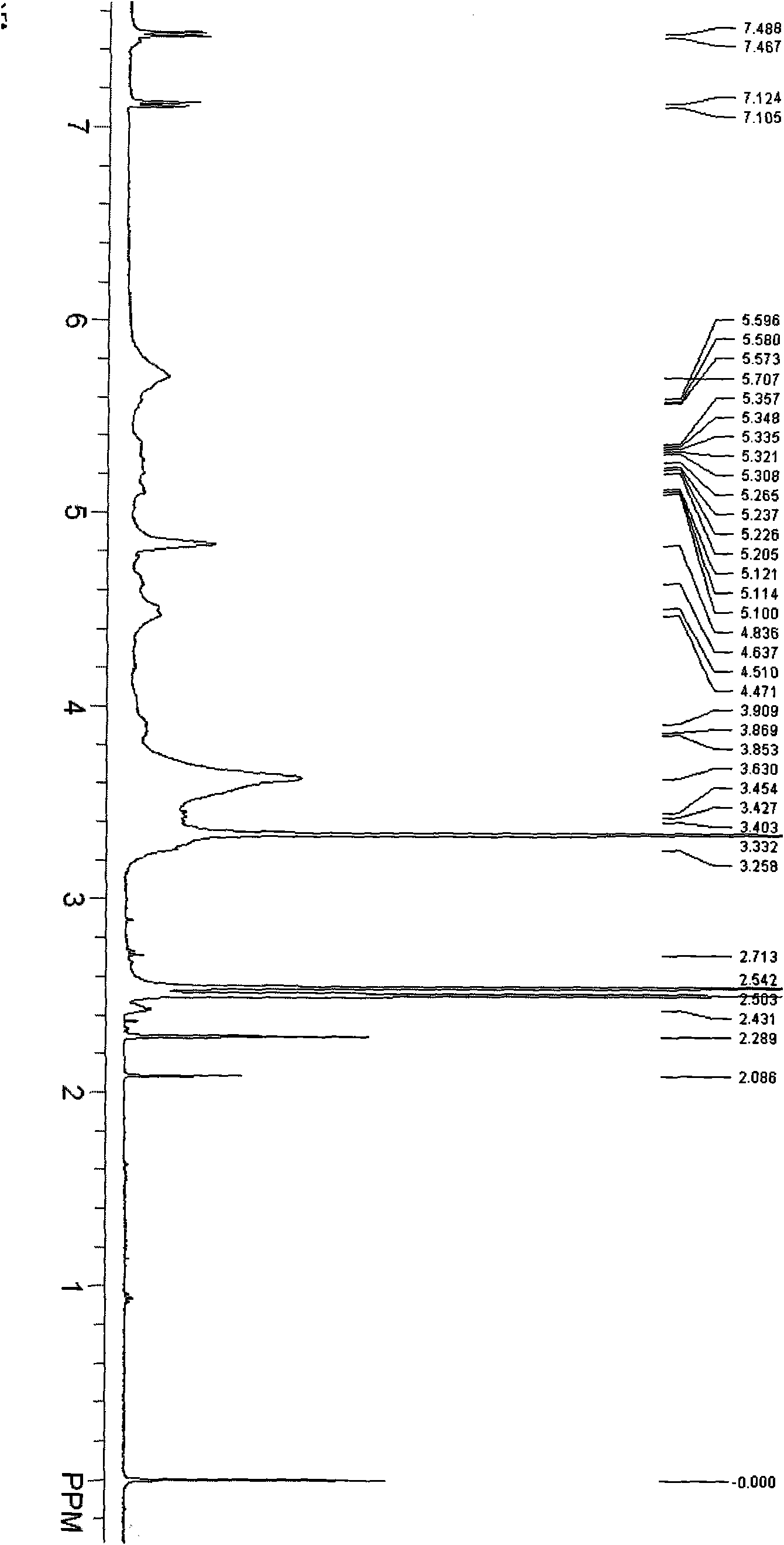
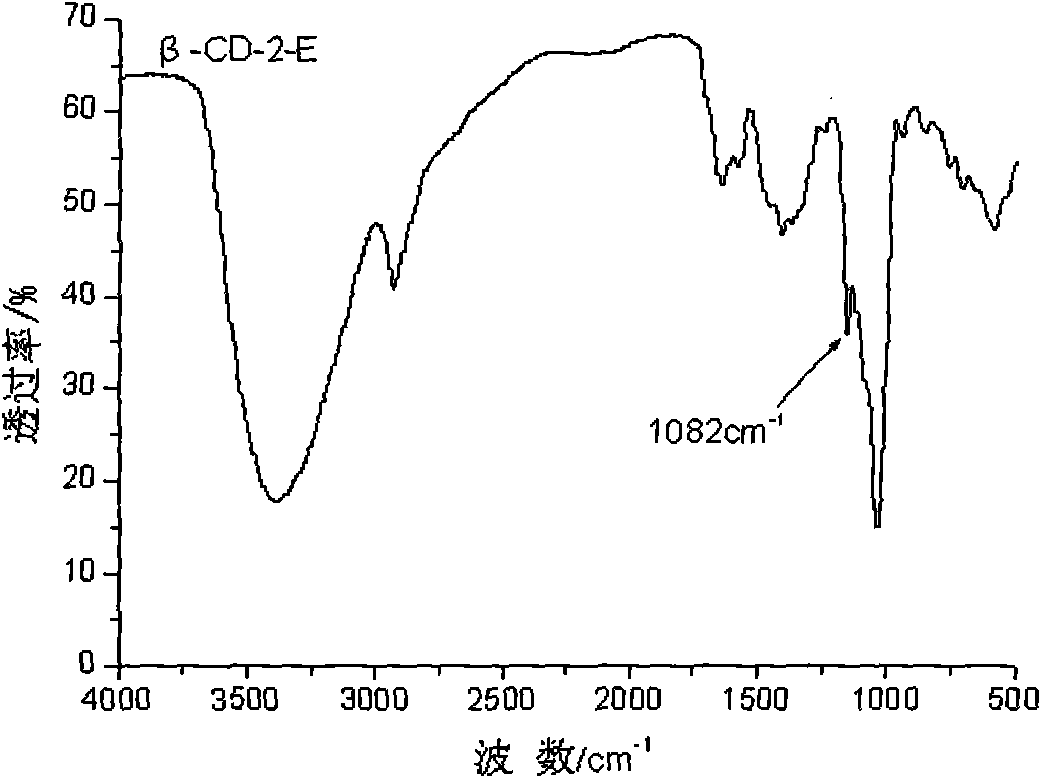
[0033] Synthesis of 2-ethylenediamine-β-cyclodextrin (...
Embodiment 2
[0036] Synthesis of 2-tosylated-β-cyclodextrin (β-CD-2-OTs): 8gβ-CD (approximately 7.06 mmol) and 300ml of 0.15mol / L NaOH aqueous solution, fully stirred to dissolve. Dissolve 8 g of p-toluenesulfonyl chloride in a small amount of acetonitrile, and slowly drop it into the three-necked flask within 3 hours. During the dropping process, 1 mol / L NaOH aqueous solution is continuously added to ensure that the pH of the reaction solution is ≥ 12.5. After p-toluenesulfonyl chloride was dropped, the stirring reaction was continued for 3 hours. After the reaction is completed, neutralize to neutral with 1mol / L hydrochloric acid, filter off a small amount of unreacted p-toluenesulfonyl chloride. Vacuum rotary evaporate the filtrate to dryness, and obtain a white solid. Then desalt the solid twice with 200ml of anhydrous methanol, reflux The liquid is cooled, filtered, and evaporated to obtain the white powder product β-CD-2-OTs.
[0037] Synthesis of 2-ethylenediamine-β-cyclodextrin (...
Embodiment 3
[0040] Synthesis of 2-tosylated-β-cyclodextrin (β-CD-2-OTs): 8gβ-CD (approximately 7.06 mmol) and 300ml of 0.15mol / L NaOH aqueous solution, fully stirred to dissolve. Dissolve 10 g of p-toluenesulfonyl chloride in a small amount of acetonitrile, and slowly drop it into the three-necked flask within 4 hours, and continuously replenish 1 mol / L NaOH aqueous solution during the dropping process to ensure that the pH of the reaction solution is ≥ 12.5. After p-toluenesulfonyl chloride was dropped, the stirring reaction was continued for 4 hours. After the reaction is completed, neutralize to neutral with 1mol / L hydrochloric acid, filter off a small amount of unreacted p-toluenesulfonyl chloride. Vacuum rotary evaporate the filtrate to dryness, and obtain a white solid. Then desalt the solid twice with 200ml of anhydrous methanol, reflux The liquid is cooled, filtered, and evaporated to obtain the white powder product β-CD-2-OTs.
[0041] Synthesis of 2-ethylenediamine-β-cyclodext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com