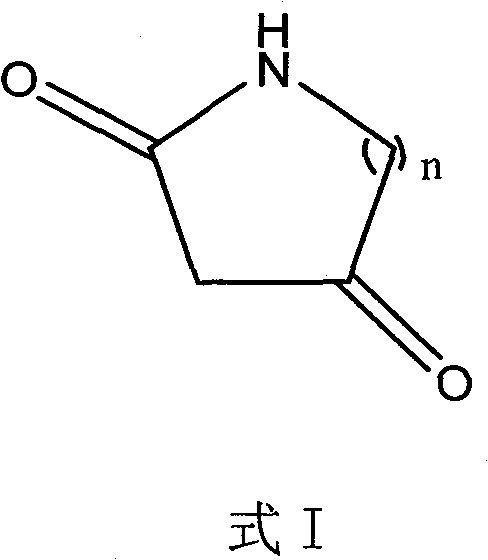
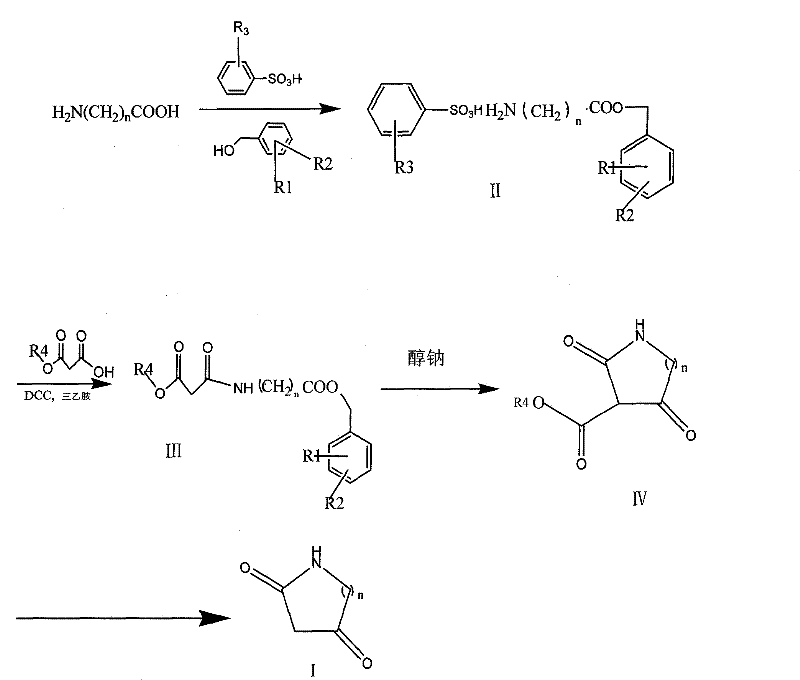
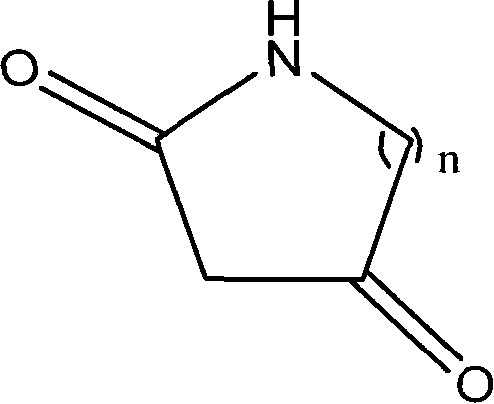
Method for synthesizing diketo nitrogen-containing compound and intermediates thereof
A synthetic method, methyl technology, applied in the field of synthesis of nitrogen-containing diketone compounds with five-membered rings and six-membered rings, which can solve the problems of affecting the reaction yield, difficult to dry out, cumbersome operation, etc., and achieves high yield and easy operation , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 2, the preparation of 4-piperidine diketone
[0037] 1. Preparation of β-alanine benzyl ester p-toluenesulfonate:
[0038] Add 200g of β-alanine, 465g of p-toluenesulfonic acid, 970g of benzyl alcohol and 4L of toluene in sequence into a 10L three-necked reaction flask, stir, the reaction solution becomes turbid, heated in an oil bath, dissolves, and the reaction solution becomes a clear and transparent colorless solution; Heat to reflux to separate water, judge the end point of the reaction with TLC (developing agent CHCl 3 :CH 3 OH=5:2 Rf=0.56). Add 1200ml of toluene and leave it overnight at room temperature (16°C). A large number of flaky crystals were precipitated, and the filter cake was fully washed by filtering 3×500 ml of petroleum ether, and dried under reduced pressure at 80° C. to obtain 773 g of flaky white crystals, with a yield of 98.0%. HPLC analysis 99.16%
[0039] 2. Preparation of N-(3-ethoxy-1,3-dioxypropyl)-beta-alanine benzyl este...
Embodiment 2
[0047] Example 2 Preparation of 2,4-tetrahydropyrrole diketone
[0048] 1. Preparation of glycine p-methylbenzyl benzenesulfonate
[0049] Add 150g of glycine, 344g of benzenesulfonic acid, 860g of p-methylbenzyl alcohol and 3.5L of toluene into a 5L three-necked reaction flask in sequence, stir, the reaction solution becomes turbid, heated in an oil bath, dissolves, and the reaction solution becomes a clear and transparent colorless solution; Heat to reflux to separate water, judge the end point of the reaction with TLC (developing agent CHCl 3 :CH 3 OH=5:2 Rf=0.55). Add 1100ml of toluene and leave it overnight at room temperature (16°C). A large number of crystals were precipitated, and the filter cake was fully washed by filtering 3×500ml of petroleum ether, and dried under reduced pressure at 80°C to obtain 665g of flaky white crystals, the yield was 98.7%, and the HPLC analysis was 99.42%
[0050] 2. Preparation of N-(3-methoxy-1,3-dioxypropyl)-glycine p-methylbenzyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com