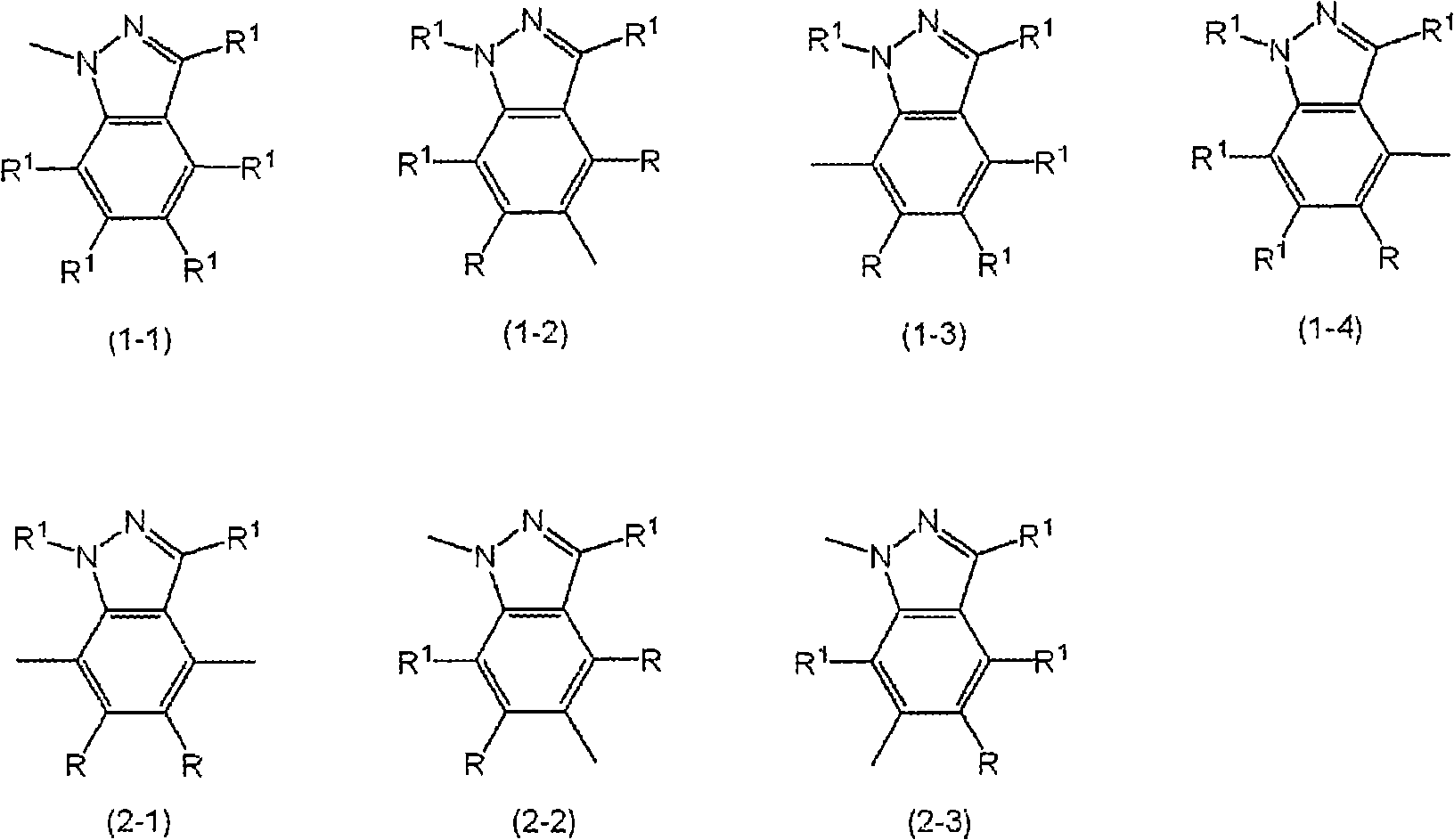
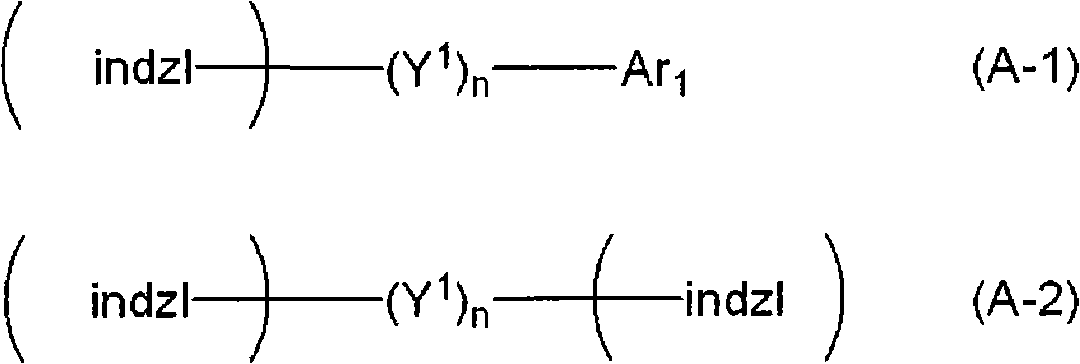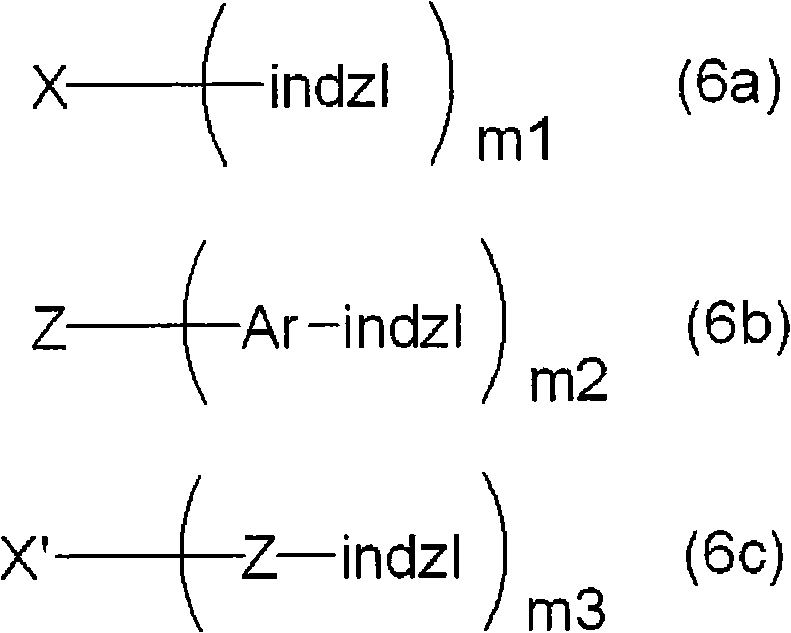Indazole compound-containing composition and light-emitting device using the composition
A technology of light-emitting elements and compounds, applied in electrical components, light-emitting materials, organic chemistry, etc., can solve the problems of low triplet excitation energy and low luminous efficiency of light-emitting materials, and achieve high triplet excitation energy, excellent luminous efficiency, and luminous efficiency. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] The minimum triplet excitation energy T as an extrapolated value in n=∞ of the polymer (P-1) represented by the following formula 1 (1 / n=0) is 3.1eV, the absolute value of the energy level E of the lowest unoccupied molecular orbital energy level LUMO (1 / n=0) is 1.8eV, and the minimum dihedral angle is 79°.
[0154]
[0155] (In the formula, n is the aggregation number.)
[0156] The calculation of the parameters is carried out using computational science methods described in the detailed description of the invention. Specifically, using the following repeating unit (M-1) in the polymer (P-1), for the cases of n=1, 2 and 3, the structure was optimized by the HF method.
[0157]
[0158] At this time, as a basis function, use 6-31G * . Then, using the same basis function, the energy level of the lowest unoccupied molecular orbital energy level and the lowest triplet excitation energy were calculated by B3P86-level time-dependent density functional method. The ...
Embodiment 2
[0162] A THF solution (about 1% by weight) of a compound represented by the following formula (C-1) was mixed at about 5 times the weight of a THF solution (0.05% by weight) of a phosphorescent compound (MC-1) represented by the following formula ). 10 µl of the resulting solution was dropped on a slide glass and air-dried to obtain a solid film. It was irradiated with ultraviolet rays of 365 nm, and green light emission from the above-mentioned phosphorescent compound (MC-1) was confirmed.
[0163]
[0164] In addition, the compound represented by the above-mentioned formula (6-1) was synthesized according to the method described in JP-A-2004-292432.
Embodiment 3
[0166] About 5 times the weight of a THF solution (about 1% by weight) of the compound represented by the following formula (C-3) was mixed with the THF solution (0.05% by weight) of the phosphorescent compound (MC-1). 10 µl of the resulting solution was dropped on a slide glass and air-dried to obtain a solid film. It was irradiated with ultraviolet rays of 365 nm, and green light emission from the above-mentioned phosphorescent compound (MC-1) was confirmed.
[0167]
[0168] The lowest triplet excitation energy T of the compound shown in the above formula (C-3) 1 is 3.1eV, the absolute value E of the energy level of the lowest unoccupied molecular orbital LUMO is 1.7eV. The dihedral angle between the indazole ring and the partial structure adjacent to the indazole ring (benzene ring in this example) was 38°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com