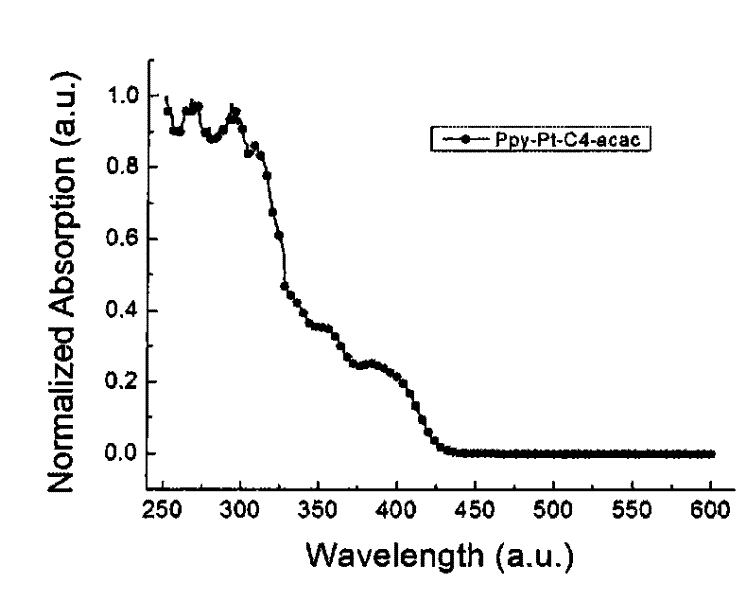
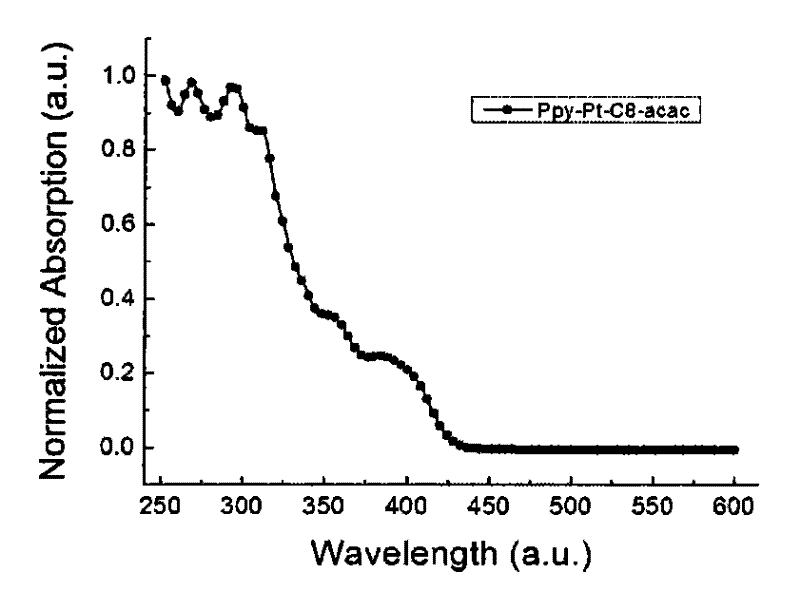
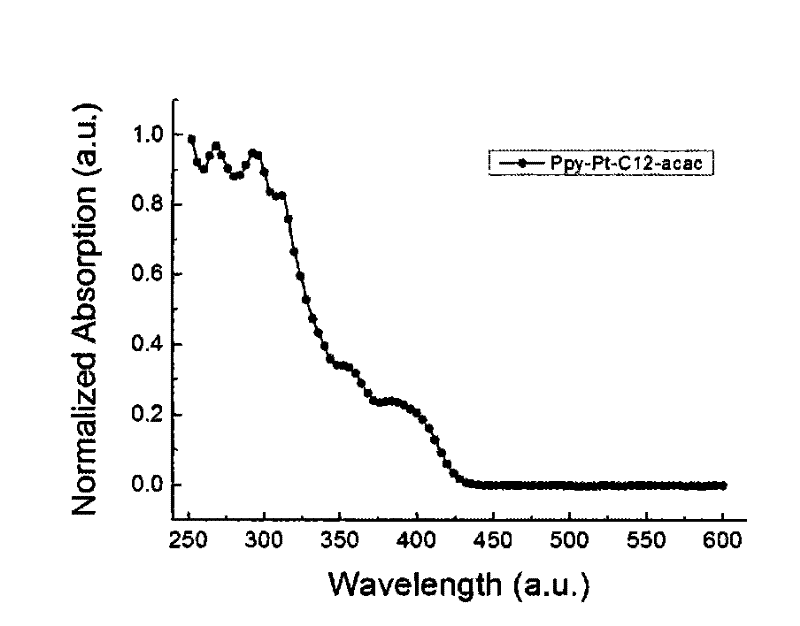
Cyclic metal platinum complex liquid crystal polarized luminescent material and its application
A technology of platinum complexes and luminescent materials, which is applied in the field of liquid crystal polarized luminescent materials and cyclometal platinum complexes, can solve the problems of low luminous efficiency and luminous brightness, no literature reports, unfavorable liquid crystal backlight, etc., and achieve high luminescence Efficiency, ease of synthesis and purification, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of 2-(4'-alkoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane
[0069]
[0070] 1.1 Synthesis of 4-butoxybromobenzene
[0071] In a 250mL single-necked bottle, add 6.9g (39.6mol) p-bromophenol, 7.9g (57.3mol) 1-bromobutane, 15.0g (108.7mol) anhydrous potassium carbonate, 0.5g (4.2mol) potassium iodide and 100mL acetone , at N 2 Reflux for 24h under atmosphere. Cool, filter with suction, and remove acetone under reduced pressure. The residue is separated by column chromatography using petroleum ether as the eluent to obtain 7.5 g of a colorless liquid with a yield of 82.9%. 1 H NMR (400MHz, CDCl 3 , TMS), δ (ppm): 7.37-7.34 (m, 2H), 6.79-6.76 (m, 2H), 3.93-3.90 (t, 2H), 1.79-1.72 (m, 2H), 1.53-1.43 (m , 2H), 0.99-0.95(t, 3H).
[0072] 1.2 Synthesis of 4-octyloxybromobenzene
[0073] The method is the same as 1.1 to obtain a colorless liquid with a yield of 82.9%. 1 H NMR (400MHz, CDCl 3 , TMS), δ (ppm): 7.37-7.34 (m, 2H), 6.79-6.76 (m, 2H), 3.92-3.89 ...
Embodiment 2
[0087] Synthesis of 2-(3'-fluoro-4'-alkoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane
[0088]
[0089] 2.1 Synthesis of 3-fluoro-4-octyloxybromobenzene
[0090] In a 250mL three-necked flask, add 5.0g (26.2mmol) 2-fluoro-4-bromophenol, 6.0g (31.1mmol) 1-bromo-n-octane, 15.0g (108.7mmol) anhydrous K 2 CO 3 , a catalytic amount of potassium iodide and 80 mL of acetone were refluxed for 24 h under the protection of nitrogen. Cool, filter with suction, and remove acetone under reduced pressure. The residue is separated by column chromatography with petroleum ether as eluent to obtain 4.8 g of colorless liquid with a yield of 60.5%. 1 H NMR (400MHz, CDCl 3 , TMS), δ (ppm): 7.25-7.22 (d, J = 12.0Hz, 1H), 7.18-7.16 (d, J = 8.0Hz, 1H), 6.86-6.82 (t, 1H), 4.02-3.99 ( t, 2H), 1.83-1.79(m, 2H), 1.465-1.29(m, 10H), 0.9-0.87(t, 3H).
[0091] 2.2 Synthesis of 3-fluoro-4-dodecyloxybromobenzene
[0092] The method is the same as 2.1 to obtain a light yellow liquid with a yield of ...
Embodiment 3
[0102] Synthesis of 2-bromo-5-(alkoxymethyl)pyridine
[0103]
[0104] 3.1 Synthesis of 2-bromo-5-pyridinecarbaldehyde
[0105] In a 500mL three-necked flask, add 6.0g (25.3mmol) of 2,5-dibromopyridine and 200mL of anhydrous ether, under nitrogen protection, at -78.5°C, slowly add 16mL (25.6mmol) of n-butyllithium (1.6mol / L), after reacting for 30min, add 2.2mL DMF dropwise, continue to react for 2h, then warm up to room temperature for 1h, add 50mL water to terminate the reaction. The reaction solution was adjusted to PH=7 with dilute HCl, distilled off ether, and then distilled off with CH 2 Cl 2 extraction. The extract was washed with water, dried over anhydrous magnesium sulfate, and separated by a column (petroleum ether: ethyl acetate = 6:1) to obtain 2.21 g of a white solid. Yield 47.0%. m.p.: 104-110°C. 1 HNMR (400MHz, CDCl 3 , TMS), 10.09(s, 1H), 8.82(s, 1H), 8.03-8.00(d, J=10.6Hz, 1H), 7.69-7.67(d, J=8.1Hz, 1H).
[0106] 3.2 Synthesis of 2-bromo-5-pyridin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com