Process for production of dibenzoxepin compound
A manufacturing method and technology of diphenyl, which is applied in the direction of organic chemistry, etc., can solve the problem of not finding the target of the efficient Z type.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
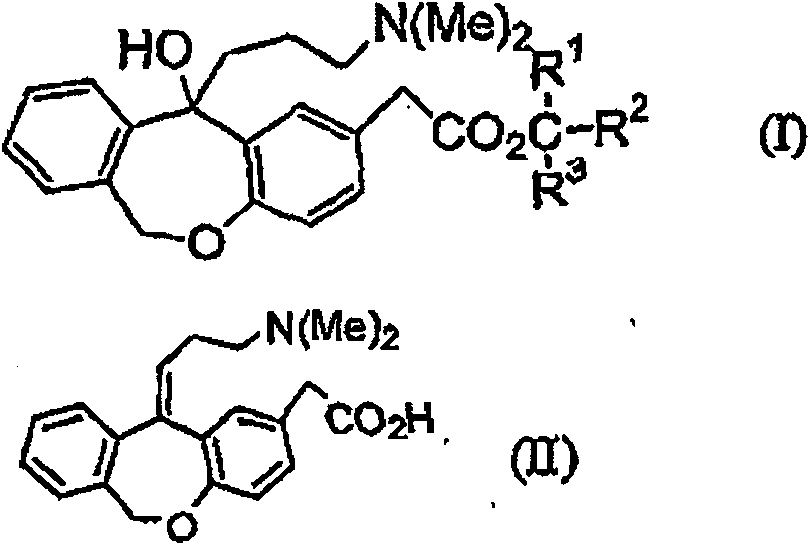
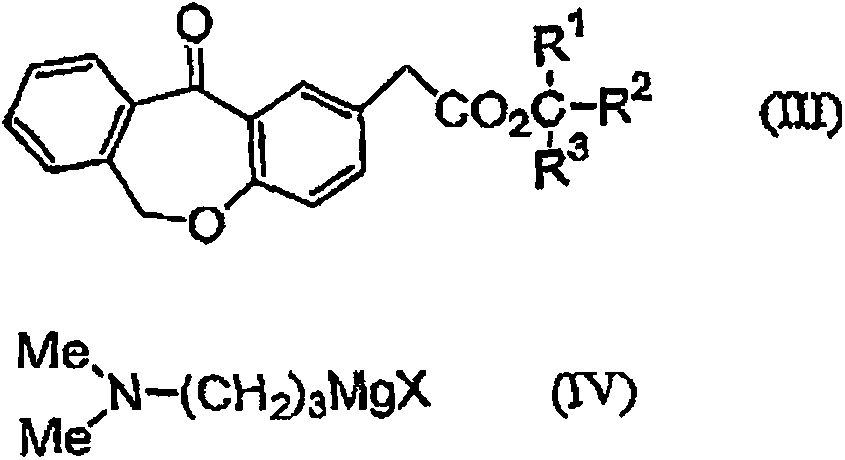
[0043] 11-Hydroxy-11-(3-dimethylaminopropyl)-6,11-dihydrodibenzo[b,e]oxa -Manufacture of 2-tert-butyl acetate
[0044] 143 g of water, 110 ml of toluene, and 146.4 g of 25% aqueous sodium hydroxide solution were added to 121.4 g (0.5 mol) of a 65.1% aqueous solution of 3-dimethylaminopropyl chloride hydrochloride, and stirred at about 25° C. for 30 minutes. 16.8 g of potassium carbonate was added to the separated organic layer, dehydrated, filtered, washed with 60 ml of toluene, and the filtrate and washing liquid were combined to prepare a toluene solution of 3-dimethylaminopropyl chloride.
[0045] 10 ml of tetrahydrofuran (THF) and 0.73 g of magnesium were mixed, and 0.1 g of 1,2-dibromoethane was added to activate the magnesium. To this, 11.6 g of toluene solutions of 3-dimethylaminopropyl chloride were dripped at 37-39 degreeC over 30 minutes. The mixture was stirred at 50° C. for 1 hour to prepare a Grignard reagent.
[0046] In dissolved with (11-oxo-6,11-dihydrodib...
Embodiment 2
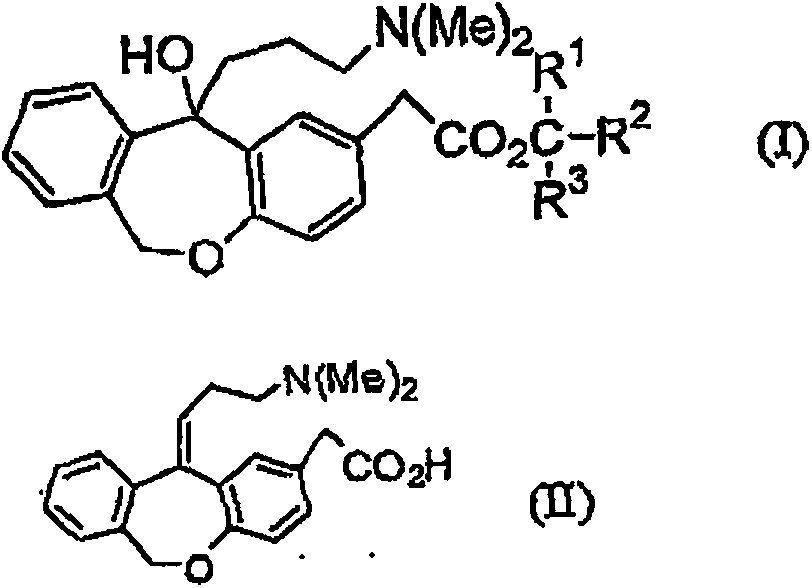
[0049] (Z)-11-oxo-(3-dimethylaminopropylidene)-6,11-dihydrodibenzo[b,e]oxa -Manufacture of 2-acetic acid hydrochloride (olopatadine hydrochloride)
[0050] Charge the flask with 11-hydroxy-11-(3-dimethylaminopropyl)-6,11-dihydrodibenzo[b,e]oxa - 10.0 g (0.0243 mol) of tert-butyl 2-acetate and 20 ml of toluene, and 3.8 g (0.03645 mol) of 35% hydrochloric acid were added. The ratio of Form E:Form Z in the reaction solution was 85:15. The reaction solution was stirred for 14 hours at a bath temperature of 100-105°C. (The internal temperature starts to reflux at 88°C, and the temperature rises to 95°C). At this moment, the E-type:Z-type ratio is 66:33. Then, a Dean-Stark apparatus was installed, and water was azeotropically distilled off (distillation removal amount: about 10 ml of toluene and about 1 ml of water). The reaction liquid was cooled to room temperature, and the solvent was removed. 100 ml of acetone and 1 ml of water were added to the residue, followed by stir...
Embodiment 3
[0060] (Z)-11-(3-Dimethylaminopropylidene)-6,11-dihydrodibenzo[b,c]oxa -2-Acetic acid hydrochloride (olopatadine hydrochloride)
[0061] In a Teflon-coated reaction kettle with a capacity of 100 ml, 11-hydroxy-11-(3-dimethylaminopropyl)-6,11-dihydrodibenzo[b,c]oxa - 5.0 g (0.0122 mol) of tert-butyl 2-acetate and 10 ml of toluene, and 0.63 g (0.0173 mol) of hydrogen chloride gas was added by bubbling at 20°C. Then, after sealing and stirring at a temperature from 90°C to 97°C for 8 hours, the reaction solution was cooled to 25°C. At this moment, the E type: Z type is 3.5:96.5. After adding 10 ml of toluene to dilute the reaction solution, the crystal was washed with 20 ml of toluene and further with 10 ml of acetone. It was dried at 50° C. to obtain 3.8 g (0.0101 mol) of olopatadine hydrochloride. The apparent yield was 83%, and the purity measured by HPLC was 97.5% for type Z and 1.1% for type E.
[0062] 1 H NMR (400MHz, DMSO-d 6 )δ2.73(s, 6H), 2.77(td, J=7.6, 7.2Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com