Synthesis and application of o-hydroxy-containing Schiff base type visible light photosensitizer with conjugated structure
A technology of conjugated structure and Schiff base type, which is applied in the synthesis and application of visible light photosensitizers, can solve the problems that the initiation efficiency needs to be improved, the dye solubility is not very good, etc., and the source of raw materials is convenient and easy to obtain, and the large molar extinction Coefficients, methods of synthesis, and effects of separating simplicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
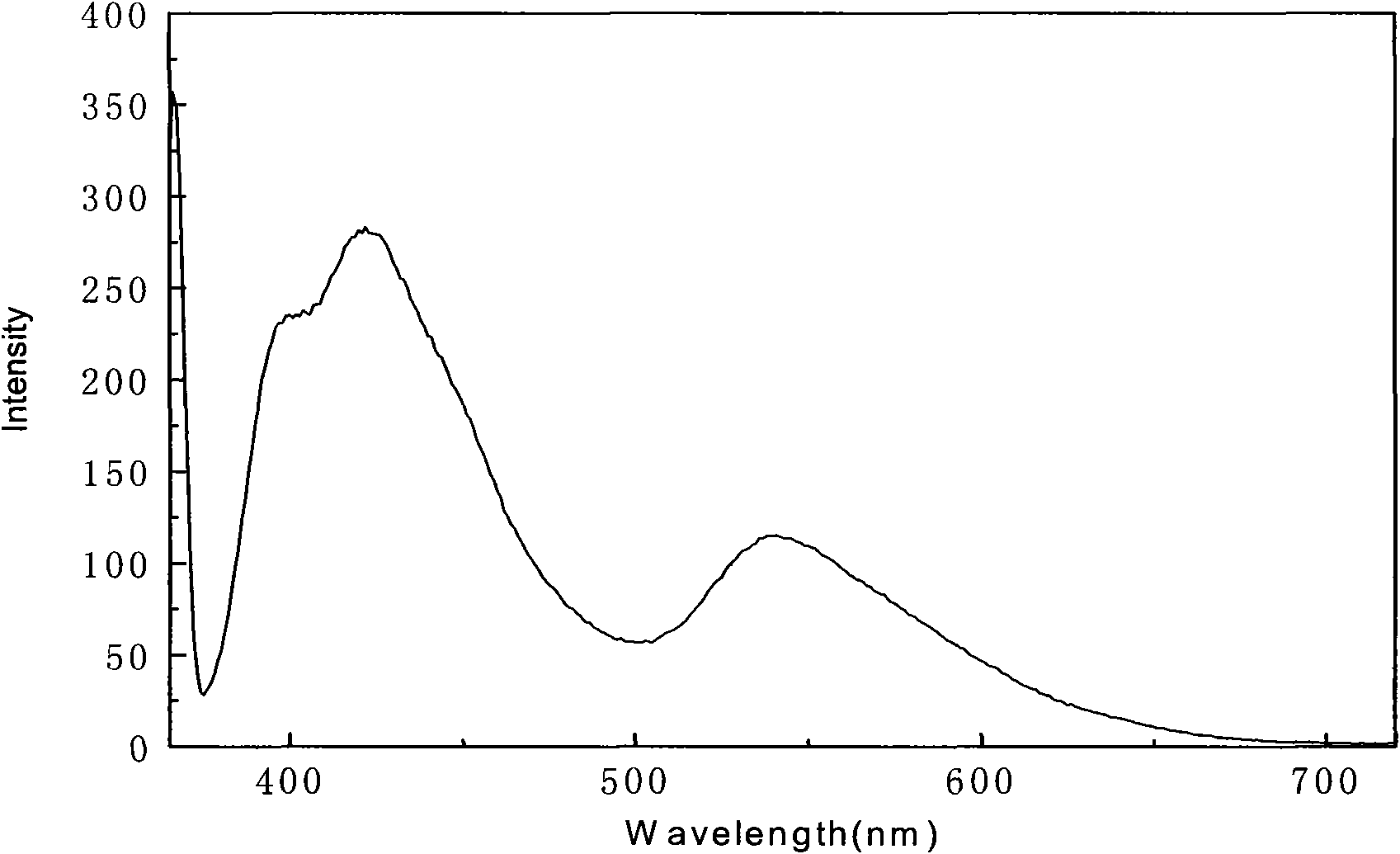
Image
Examples
Embodiment 1
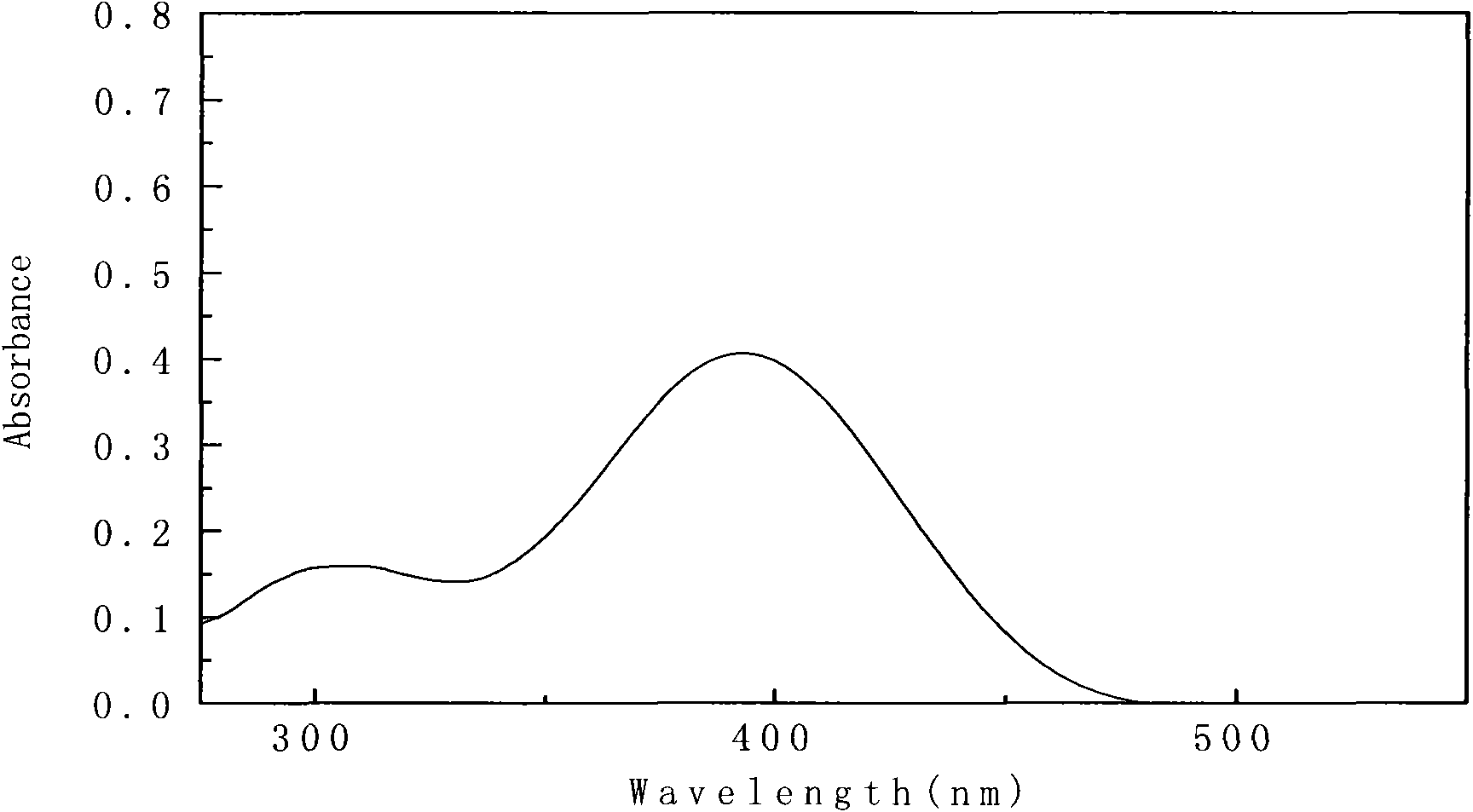
[0041] Synthesis of 2-(4-(4-(dimethylamino)styryl)phenylimino)methyl)phenol
[0042] The synthesis proceeds in three steps:
[0043] (1) N,N-Dimethyl-4-(4-nitrostyryl)aniline
[0044] Put 1.8222g of p-nitrophenylacetic acid and 1.000g of p-dimethylaminobenzaldehyde into a three-necked flask with a molar ratio of 1.5:1 and mix them, add a condensing device, and add 0.847g (about 1ml) of hexahydrogen dropwise under constant stirring For pyridine, reflux at 100°C for 3 hours, then raise the temperature to 130°C and continue to reflux for 4-5 hours until no bubbles come out and stop the reaction. The solid obtained after the reaction was recrystallized twice with absolute ethanol, and dried in a vacuum oven for further use, with a yield of 95%.
[0045] (2) Synthesis of 4-(4-aminostyryl)-N,N-dimethylaniline
[0046] Add 0.500 g of the first step product N, N-dimethyl-4-(4-nitrostyryl) aniline and 0.842 g of stannous chloride dihydrate into a three-necked flask in a molar ratio ...
Embodiment 2
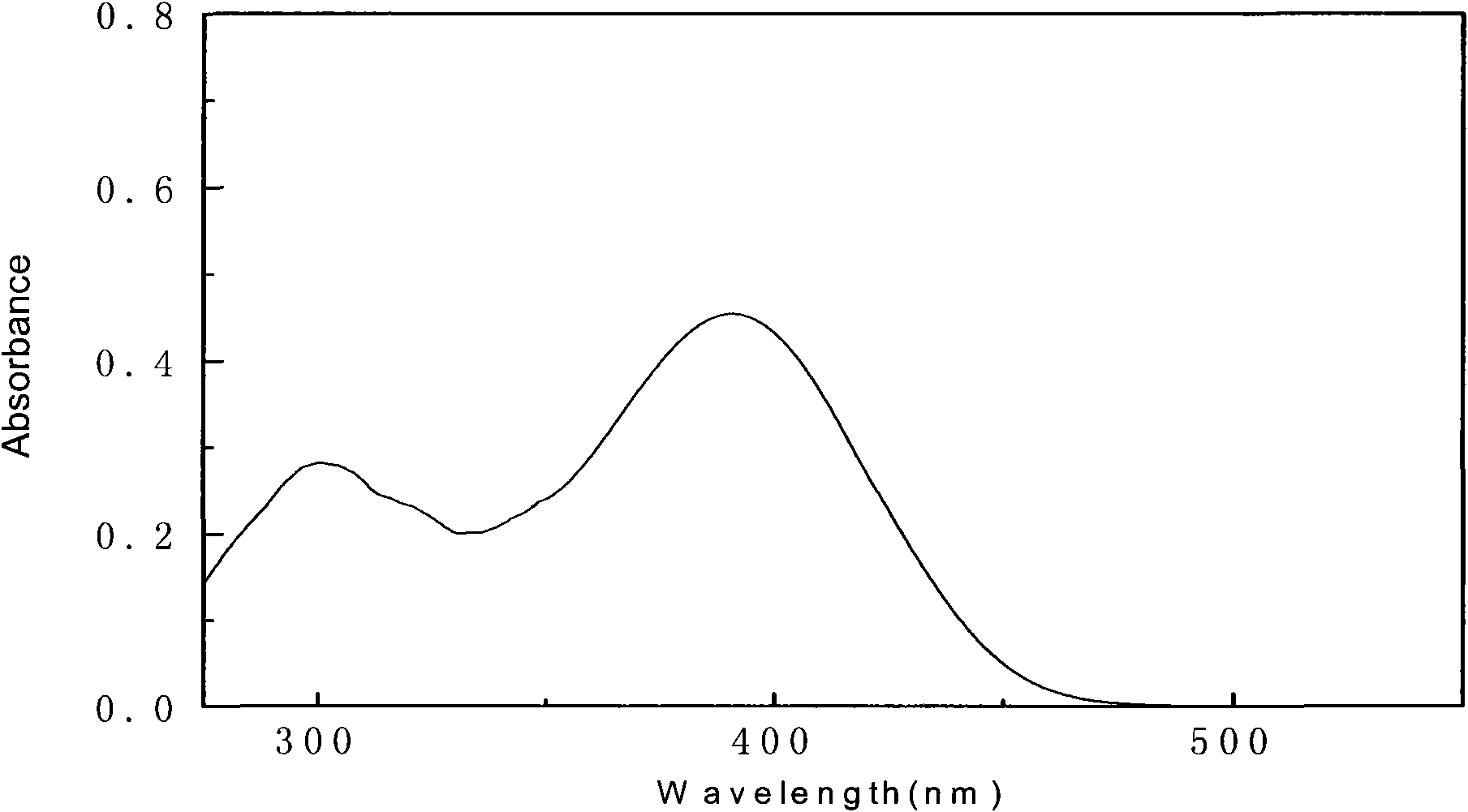
[0050] Synthesis of 2-(4-(4-(diphenylamino)styryl)phenylimino)methyl)phenol
[0051] The synthesis proceeds in three steps:
[0052] (1) 4-(4-nitrostyryl)-N,N-diphenylaniline
[0053] 0.980g of p-nitrophenylacetic acid and 1.000g of p-4-(diphenylamino)benzaldehyde were mixed in a three-necked flask with a molar ratio of 1.5:1, and a condensing device was added, and 0.847g ( About 1ml) hexahydropyridine, reflux at 100°C for 3 hours, then raise the temperature to 130°C and continue to reflux for 4 to 5 hours until no bubbles come out to stop the reaction. The dark red solid obtained after the reaction was recrystallized twice with absolute ethanol, and dried in a vacuum oven for further use. The yield was 98%.
[0054] (2) 4-N, the synthesis of N-diphenyl-(4-aminostyryl) aniline
[0055] Add 0.500 g of the first step product 4-(4-nitrostyryl)-N,N-diphenylaniline and 0.812 g of stannous chloride dihydrate into a three-neck flask in a molar ratio of 1:2 and mix , under continu...
Embodiment 3
[0059] Synthesis of 2-(4-(4-(3,4,5-trimethoxystyryl)phenylimino)methyl)phenol
[0060] The synthesis proceeds in three steps:
[0061] (1) Synthesis of 1,2,3-trimethoxy-5-(4-nitrostyryl)aniline
[0062] Add 1.385g of p-nitrophenylacetic acid and 1.000g of 3,4,5-trimethoxybenzaldehyde in a molar ratio of 1.5:1 and mix them in a three-necked flask, add a condensing device, and add 1.271g (approx. 1.5ml) hexahydropyridine, reflux at 100°C for 3 hours, then raise the temperature to 130°C and continue to reflux for 4 to 5 hours until no bubbles emerge to stop the reaction. The yellow solid obtained after the reaction was recrystallized twice with absolute ethanol, and dried in a vacuum oven for future use. The yield was 98%.
[0063] (2) Synthesis of 5-(4-aminostyryl)-1,2,3-trimethoxyaniline
[0064] Add 0.500 g of the first step product 1,2,3-trimethoxy-5-(4-nitrostyryl) aniline and 0.789 g of stannous chloride dihydrate into the three-necked flask in a molar ratio of 1:2 Mix ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com