Agent for prophylaxis or treatment of substance abuse and dependence
A medicament and alcohol technology, which is applied in the direction of drug combination, medical formula, medical preparations containing active ingredients, etc., can solve the problems of reducing alcohol consumption and increasing alcohol consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0102] Experimental Example 1: Effect on Alcohol Withdrawal Syndrome in Wistar Rats
[0103] (experimental method)
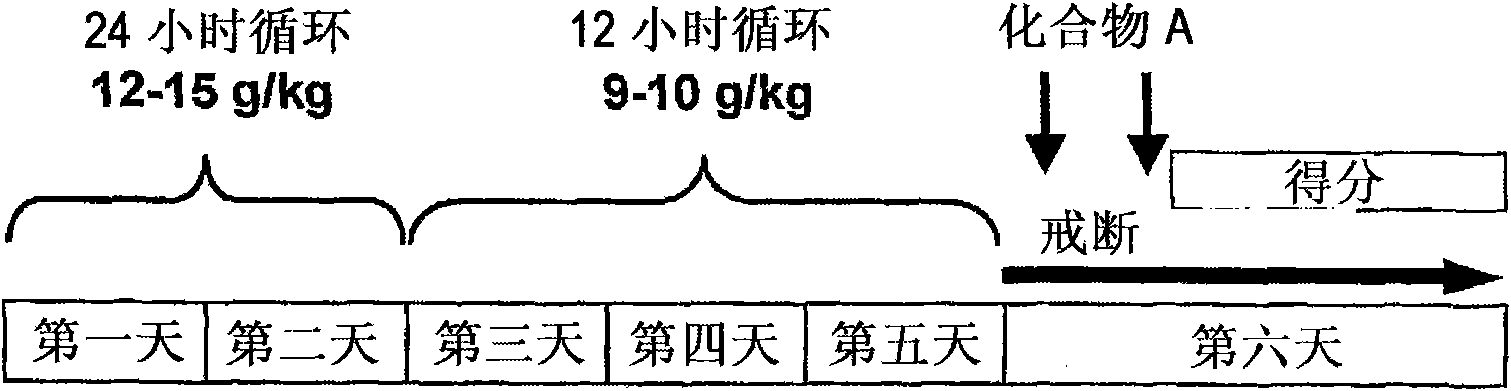
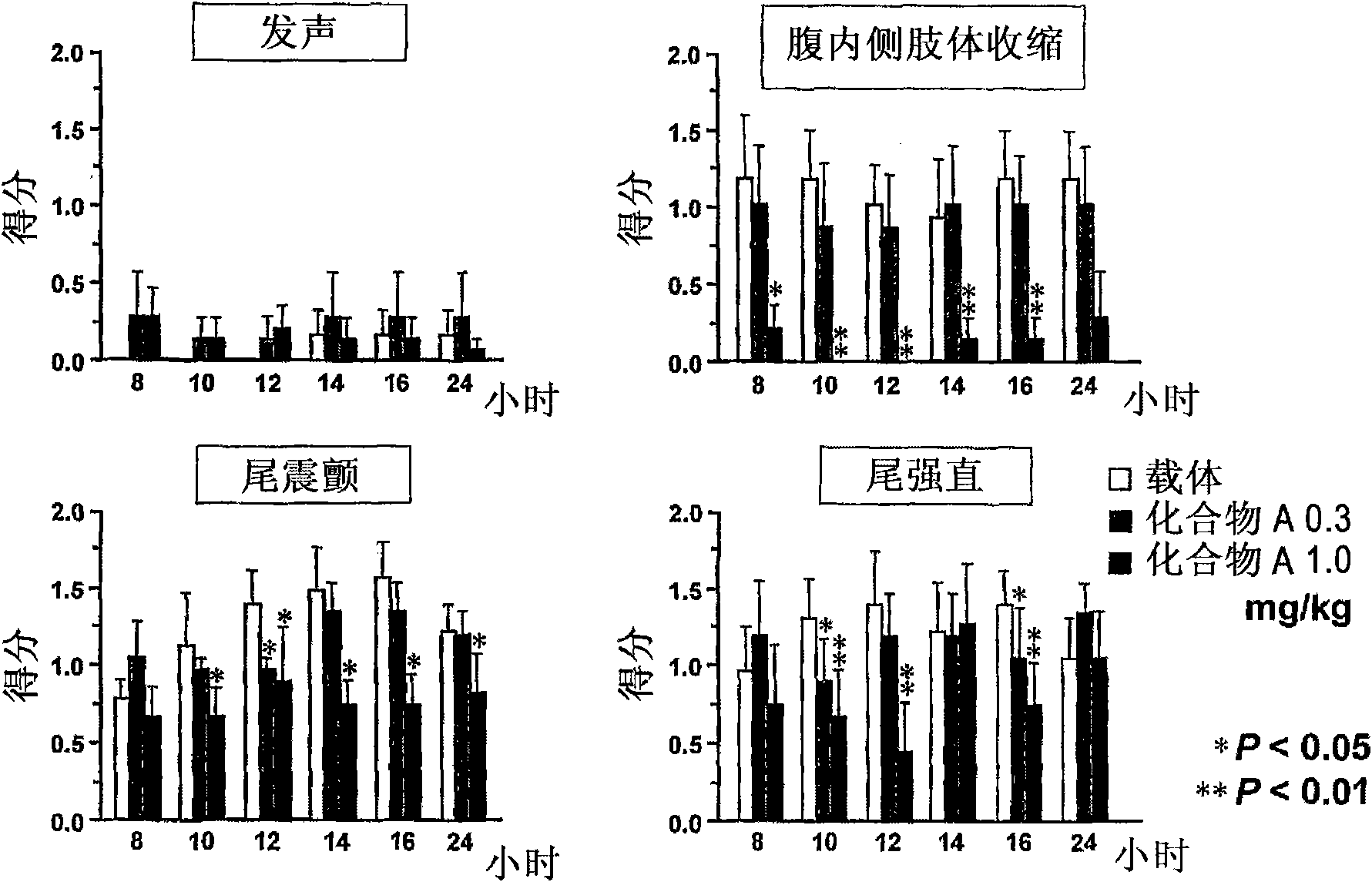
[0104] Male Wistar rats were used in this study. according to figure 1 12-15 g / kg / body weight of alcohol was orally administered to rats within 24 hours on the first day to the second day, and within 12 hours on the third to fifth day 9-10g / kg / body weight of alcohol. 2 hours and 7 hours after the last alcohol administration, rats were orally administered 0.3 mg / kg / body weight and 1 mg / kg / body weight of Compound A, respectively. Four types of alcohol withdrawal syndrome were observed, such as vocalization, ventromedial limb retraction, tail rigidity, and tail tremor, and scored according to the following 3-level rating scale: mild=0, moderate=1, severe=3.
[0105] (result)
[0106] Such as figure 2 As shown, 0.3 mg / kg / body weight and 1 mg / kg / body weight of Compound A significantly and dose-dependently reduced ventromedial limb contraction, tail rigidity an...
experiment example 2
[0107] Experimental Example 2: Effect on alcohol intake in msP rats.
[0108] (experimental method)
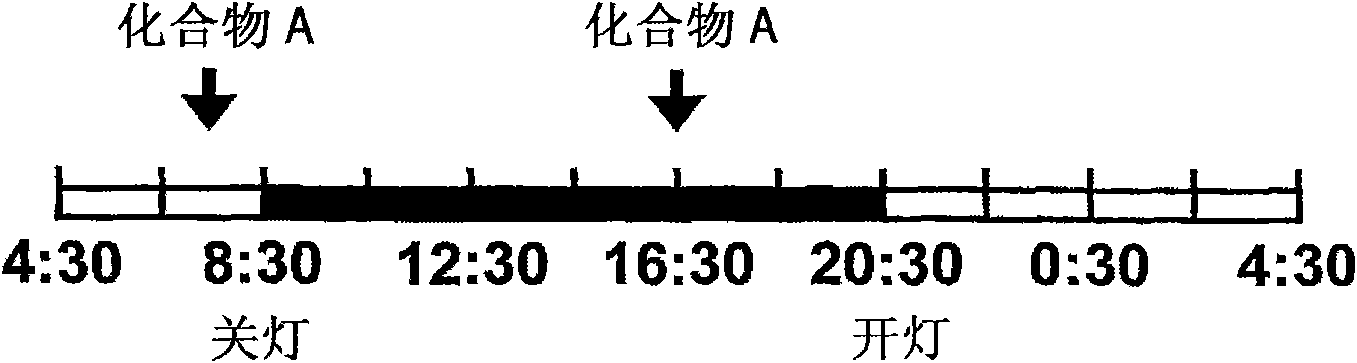
[0109] Genetically selected male alcohol-preferring rats called Marchigian Sardinian alcohol-preferring (msP) rats (Addiction Biol. 11, 339-355, 2006) were used in this study. Such as image 3 As shown, rats were housed in a day and night reversed environment (lights off at 8:30 and lights on at 20:30), which could be freely selected between water and 10% alcohol, and alcohol consumption was measured every day. One hour before the beginning of the dark period and 8 hours after the beginning of the dark period, Compound A was orally administered at 0.3 mg / kg / body weight and 1 mg / kg / body weight, respectively, for 9 consecutive days.
[0110] (result)
[0111] Such as Figure 4 As shown, Compound A at 0.3 mg / kg / body weight and 1 mg / kg / body weight significantly and dose-dependently reduced alcohol consumption in msP rats. The effect was also persistent and significant up to 9...
experiment example 3
[0112] Experimental Example 3: The effect of yohimbine-induced recovery on alcohol craving behavior in msP rats
[0113] (experimental method)
[0114] Genetically selected male alcohol-preferring rats called msP rats (Addiction Biol. 11, 339-355, 2006) were used in this study. Such as Figure 5 As shown, rats were trained to voluntarily administer 10% (w / v) alcohol per day for 30 minutes following a fixed ratio 1 (FR1) reinforcement schedule, where each response resulted in an infusion of 0.1 ml of fluid. After obtaining a stable baseline of voluntary administration of 10% alcohol, rats were subjected to extinction phases of 30 min each for 15 consecutive days. The extinction phase was the same as the voluntary 10% alcohol phase, however there was no more alcohol during the extinction phase. Starting on day ten of the extinction phase, rats were divided into three groups with similar baseline responses to 10% alcohol. For six consecutive days, each group of rats was orall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com