Screening method of anti-HIV drug
A technology of animal cells and cells, applied in biochemical equipment and methods, measurement/inspection of microorganisms, cells modified by introducing foreign genetic material, etc., can solve the problems of rapid mutation and drug resistance of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Cell Culture
[0062] 293T cells were taken for culture. After the cells filled the culture flask, the old medium was discarded and digested with a digestion solution containing 0.25% trypsin and 0.02% EDTA. When the cells become round, the digestion solution is discarded, and high-sugar DMEM medium (HyClone) containing 10% FBS (purchased from Biological industries) is immediately added, and the bottom of the bottle is gently blown with a straw to completely separate the cells from the bottom of the bottle and disperse them as single cell suspension. After counting on a hemocytometer, adjust the cell concentration to 2.2 × 10 with medium 5 cells / ml, take 15ml of cell suspension and inoculate it on a 10cm culture dish, and use it for cell transfection after 12 hours (the cell abundance is about 70%).
Embodiment 2
[0063] Example 2 Construction of expression vector
[0064] The whole gene of hA3G (GeneID: 60489) was amplified by PCR. The template is human cDNA, and the primers used are: upstream primer 5′-GCC AGA ATT CAA GGA TGA AGCCTC ACT TCA G, downstream primer 5′-TAG AAG CTC GAG GTT TTC CTGATT CTG GAG AAT GG,
[0065] reaction system:
[0066]
[0067] Reaction conditions: 98°C for 5min; 94°C / 1min, 55°C / 1min, 72°C / 1min, a total of 30 cycles; 72°C for 10min.
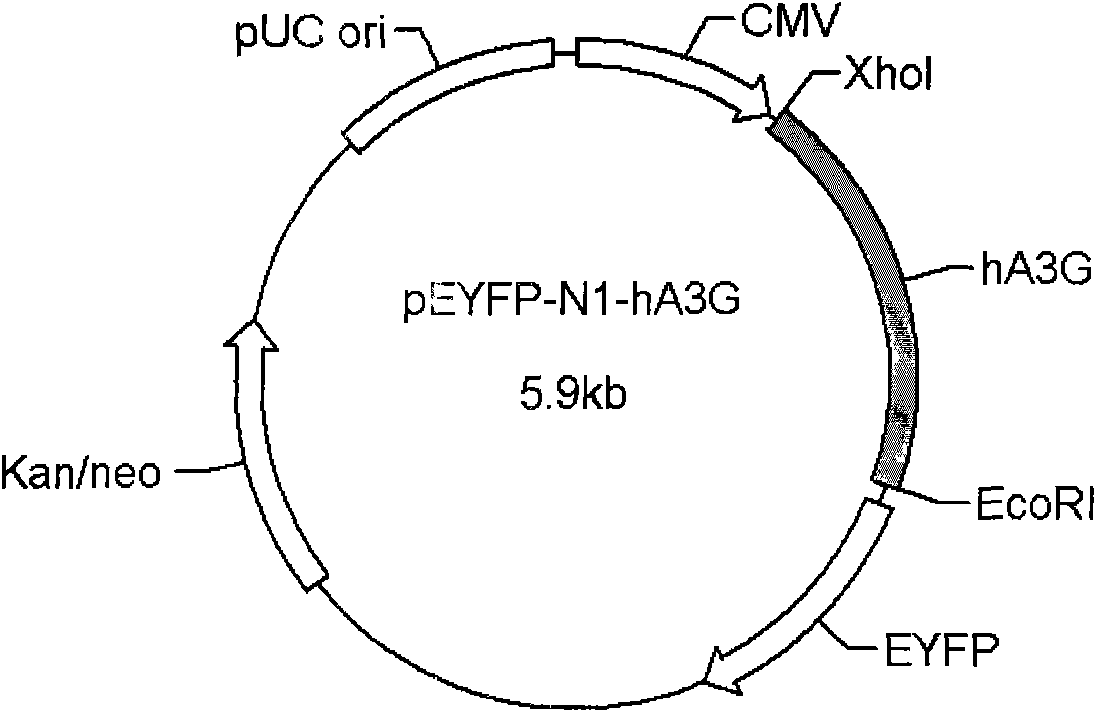
[0068] After the amplified product was verified, it was inserted into the multiple cloning site (Xho1 and EcoR1) of the eukaryotic cell expression vector pEYFP-N1 (Clontech) to obtain the plasmid pEYFP-N1-APOBEC3G. pEYFP-N1-APOBEC3G expresses the fusion protein of hA3G and YFP.
[0069] The full-length Vif gene (GeneID: 326389) was amplified by PCR from wild-type HIV-1 strain pSVC21.BH10 (NIH AIDS Research & Reference Reagent Program). Primers used:
[0070] Upstream primer 5′-TAG AAG GAA TTC ATG GAA AAC AGA TGC
[0071...
Embodiment 3
[0076] Example 3 Cell Transfection
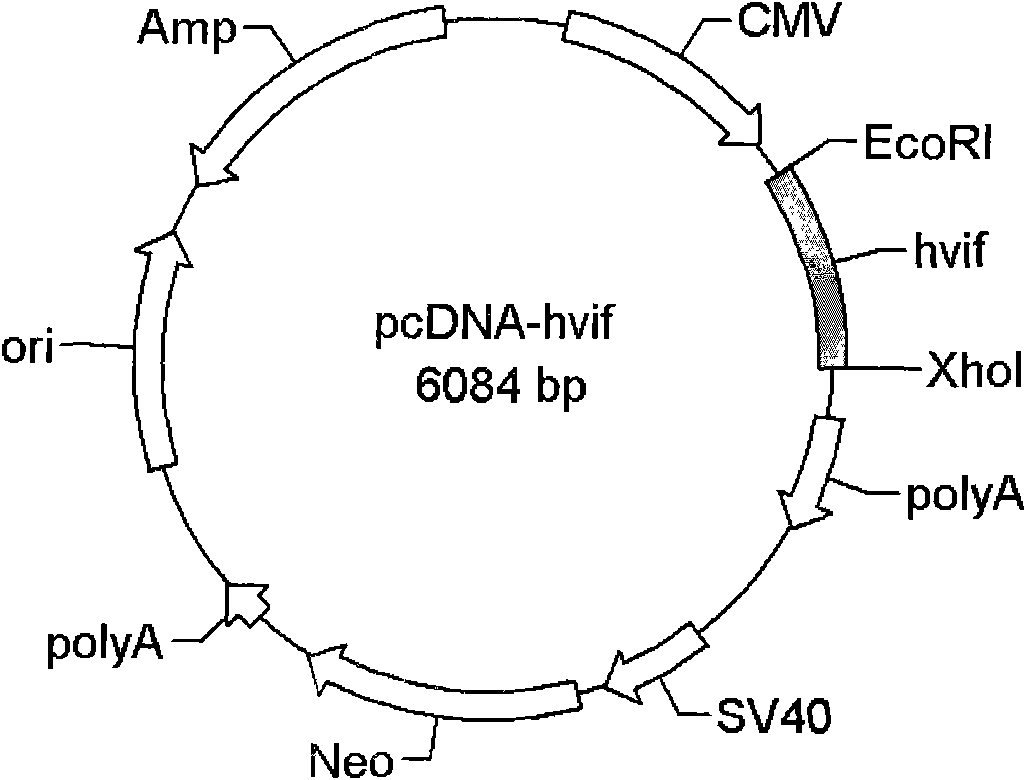
[0077] The transfection step was carried out according to the instructions of Lipofectamine 2000 (Invitrogene). The specific operation is as follows: mix the plasmid used for transfection with 1.5ml high-sugar DMEM medium, and then mix gently. Positive group: co-transfected with 12 μg pEYFP-N1-APOBEC3G and 12 μg control empty vector pcDNA3.1, experimental group: co-transfected with 12 μg pEYFP-N1-APOBEC3G and 12 μg pcDNA-hvif; blank control group: transfected with 24 μg control empty vector pcDNA3 .1.
[0078] Dilute 60 μl Lipofectamine 2000 with 1.5 ml high glucose DMEM medium. Incubate at room temperature for 5 minutes, gently mix the medium containing the transfection plasmid and Lipofectamine 2000 (total volume is 3ml), incubate at room temperature for 20 minutes, and add to the cell culture supernatant in a 10cm culture dish.
[0079] After transfection, 37 °C, 5% CO 2 Cultivate for 10h. Then suck out the old culture medium, diges...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com