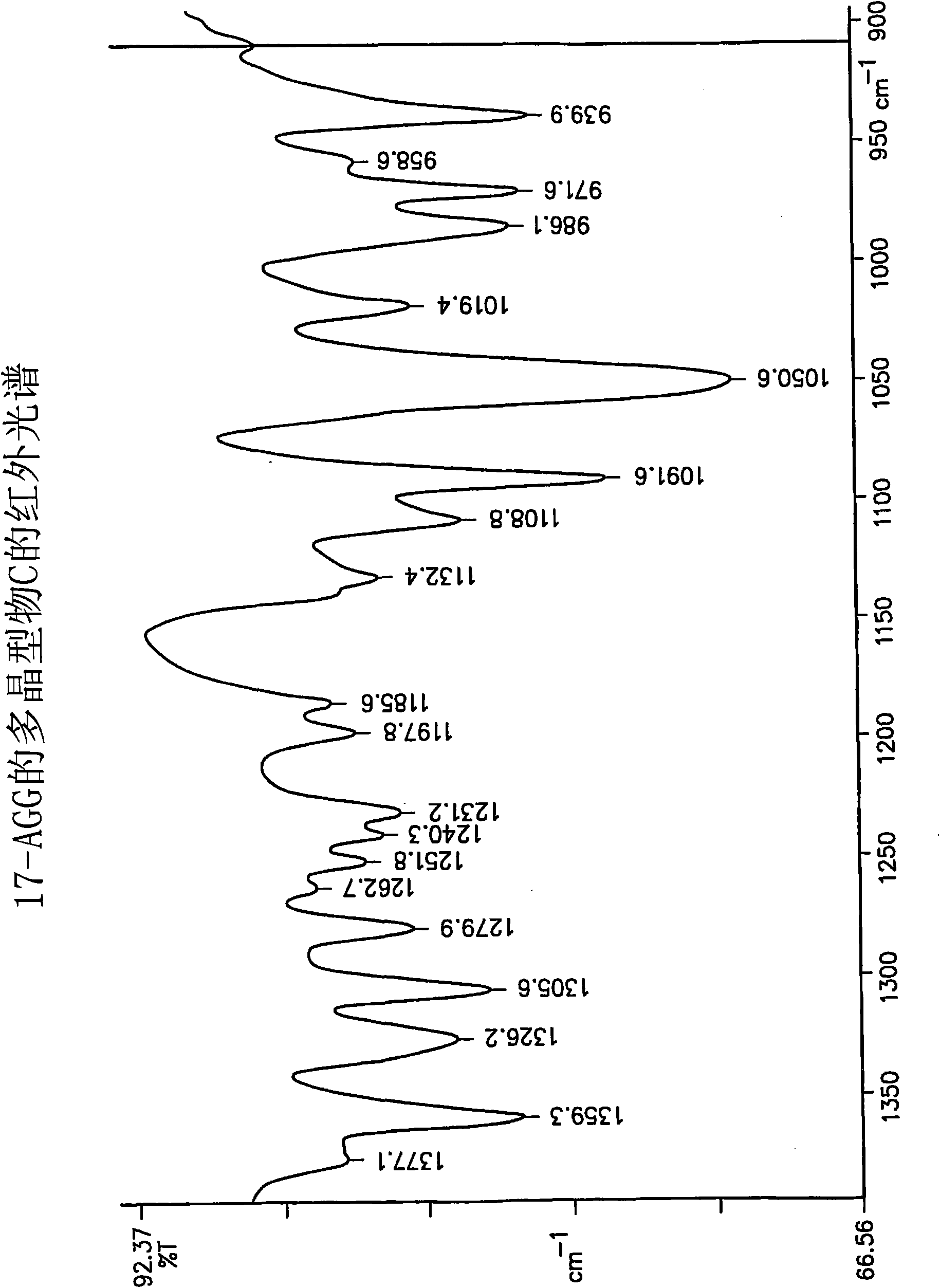
17-allylamino-17-demethoxygeldanamycin polymorphs and formulations
A technology of demethoxygeldanamycin and allylamino, applied in the field of 17-allylamino-17-demethoxygeldanamycin polymorphs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]The present method produces highly pure 17-AAG by removing polar impurities by slow crystallization from acetone-water at about ambient temperature. A flask containing crude 17-AAG (21 g) was set up for reflux. Acetone (20 mL per gram of solids) was added to the flask. The slurry was refluxed and maintained at this temperature for 5 min. The mixture was cooled to 25 °C over a period of 1 h. Water (volume equal to that of acetone) was added over a period of 1 h. After 20 min, the slurry was filtered. The filter cake was washed with 1:1 acetone:water (40 mL). The wet cake is filtered and saved for further processing. The 17-AAG thus produced has a chromatographic purity of 99.7% as polymorph B.
Embodiment 2
Example 2 - Preparation of Purified Polymorph C
[0065] This protocol yields 17-AAG consisting mainly of polymorph C with high crystallinity. If highly pure 17-AAG (as prepared according to the preceding examples) is used, polymorph C is obtained both highly pure and highly crystalline.
[0066] A solution of 17-AAG (1 g, purified according to Example 1) in acetone (100 mL) was refluxed. Water (100 mL) was added at a rate to keep the tank at or near reflux. Acetone was distilled off until the pot temperature reached 100°C. Additional fractions (20 mL, mostly water) were collected. The tank contents were cooled and the solids were collected by filtration using a Buchner funnel fitted with Whatman #52 filter paper and washed with 1:1 acetone:water (20 mL). The crystals were vacuum dried at >20°C for >2h, sampled, and vacuum dried at 85°C for approximately 12h to yield polymorph C.
Embodiment 3
Example 3 - Preparation of Purified Polymorph G
[0067] A solution of 17-AAG (10.0 g) in acetone (750 mL) was poured into water (1.05 L) with stirring at room temperature. The solution was stirred for another 70 min. Polymorph G crystals were collected by filtration and dried at 45°C for 18h.
[0068] In an alternative method, 17-AAG (1.0 g) was dissolved in acetone (117 mL) and stirred at room temperature. Water (117 mL) was added at a rate of 15 mL / min. The mixture was stirred for an additional 50 min, and polymorph G crystals were collected by filtration and dried at 70° C. for 44 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com