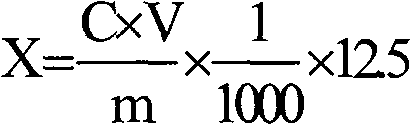Method for measuring contents of cationic surface active substances by bromothymol blue spectrometry
A technology for surface active substances, bromothymol blue, is applied in the field of bromothymol blue spectroscopic method for determining the content of cationic surface active substances, and achieves the effects of good reproducibility, high accuracy and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a volumetric flask 25mL, 50mL
[0036] b measuring cylinder 10mL
[0037] c Balance capacity 220g, sensitivity 0.1mg
[0038] d acidity meter accuracy ± 0.01
[0039] eUV-Vis spectrophotometer Photometric accuracy ±0.002Abs
[0040] 2 Reagents (all analytically pure)
[0041] a bromothymol blue reagent
[0042] b Hexadecyltrimethylammonium bromide
[0043] c sodium dihydrogen phosphate
[0044] d disodium hydrogen phosphate
[0045] ethanol
[0046] 3. Experimental steps
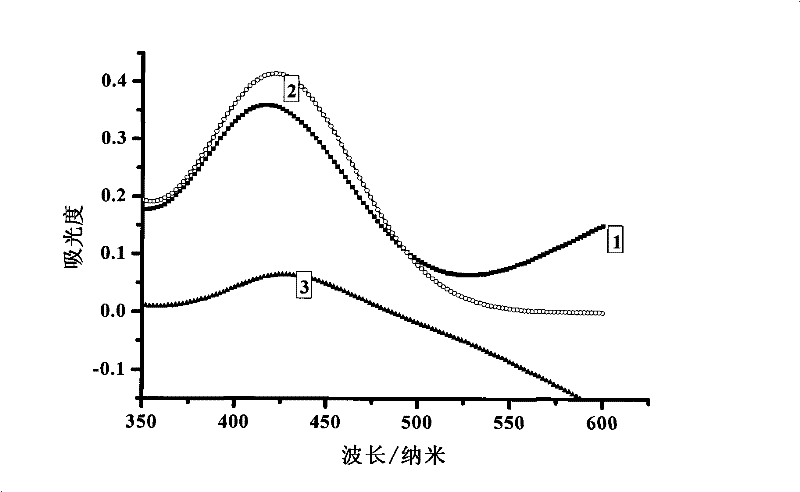
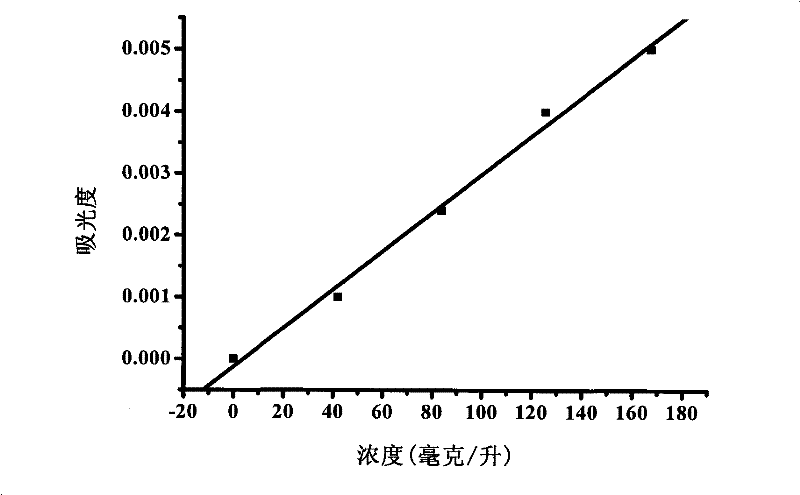
[0047] (1) Sample dissolution: Accurately weigh 2.5690 g of basic magnesium carbonate without cetyltrimethylammonium bromide and 2.5690 g of basic magnesium carbonate containing cetyltrimethylammonium bromide, two kinds The mass error of the sample was controlled within 0.2 mg. The sample was dissolved with nitric acid aqueous solution with a mass concentration of 50%, and the sample dissolution process was carried out in a closed container, and a basic magnesium carbonate solution with a conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com