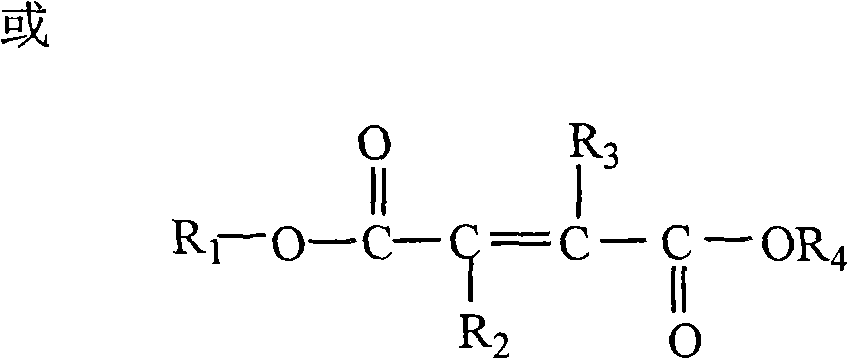
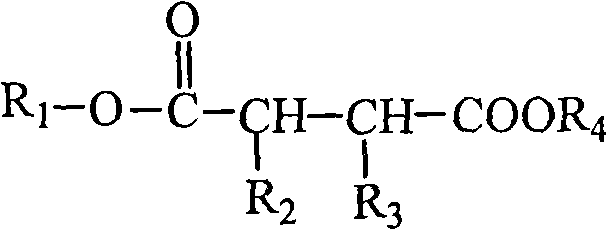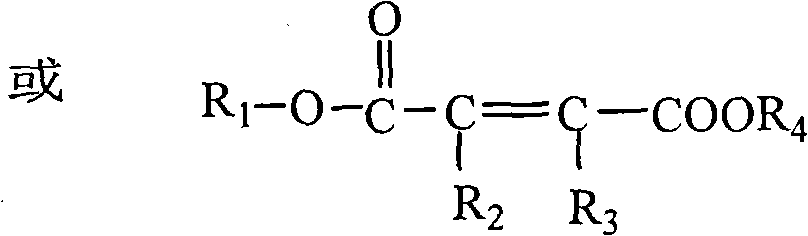Water-soluble triazole antifungal compound
A compound, triazole technology, applied in the field of water-soluble triazole antifungal compounds and its preparation, can solve problems such as safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (a) 4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy]-4- Oxobutanoic acid.
[0066]
[0067] Add 30.6g (0.1mol) of 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propanol into the three-necked flask (fluconazole), 13g (1.3mol) of succinic anhydride, 5mL triethylamine, 100mL acetone. The temperature was raised to reflux for 5 hours under stirring, and the temperature was lowered. 100 mL of water was added to the reaction mixture, crystallization was carried out under stirring, and the solid was collected by filtration. Recrystallized in 75% ethanol to obtain 31.6g of product, yield: 77.8%, [M+1] + : 407.12, H-NMR (CDCI 3 )δppm: 8.15(m, 2H), 8.11(m, 2H), 7.16(d, 1H), 6.67(d, 1H), 6.56(m, 1H), 4.26(s, 4H), 2.63(t, 2H ), 2.55(t, 2H).
[0068] (b) 4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy]-4- Sodium oxobutyrate.
[0069]
[0070] Take 20.3 g (0.05 mol) of the product from the previous step, dissolve it in 200 m...
Embodiment 2
[0072] (a) L-2-amino-4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propane Oxy]-4-oxobutanoic acid.
[0073]
[0074] Add 15g (0.11mol) of L-2-aminosuccinic acid, 150mL PCl 3 Raise the temperature to 60°C for 3 hours. Recover excess PCl under reduced pressure 3 , 250 mL of acetone was added to the residue, and 30.6 g (0.1 mol) of 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazole-1- base)-2-propanol (fluconazole), heated to reflux reaction for 5hr under stirring, cooled down, added 100mL water to the reaction mixture, crystallized under stirring, and collected the solid by filtration. Recrystallized in 75% ethanol to obtain 28.9g of product, yield: 68.6%, [M+1] + : 422.1, H-NMR (CDCI 3 )δppm: 8.17(m, 2H), 8.03(m, 2H), 7.44(d, 1H), 6.96(d, 1H), 6.35(m, 1H), 4.22(s, 4H), 4.13(t, 1H ), 2.87(d, 1H), 2.61(d, 1H).
[0075] (b) L-2-amino-4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propane Sodium oxy]-4-oxobutanoate.
[0076]
[0077]Take 21.1 ...
Embodiment 3
[0079] (a) (2Z)-4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy ]-4-oxo-2-butenoic acid.
[0080]
[0081] Add 30.6g (0.1mol) of 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propanol into the three-necked flask (fluconazole), 13g (1.3mol) of maleic anhydride, 5mL triethylamine, 100mL acetone. The temperature was raised to reflux for 5 hours under stirring, and the temperature was lowered. 100 mL of water was added to the reaction mixture, crystallization was carried out under stirring, and the solid was collected by filtration. Recrystallized in 75% ethanol to obtain 29.6g of product, yield: 73.3%, [M+1] + : 405.1, H-NMR (CDCl 3 )δppm: 8.21(m, 2H), 8.14(m, 2H), 7.25(d, 1H), 6.88(d, 1H), 6.79(m, 1H), 6.51(s, 1H), 6.35(s, 1H ), 4.31(s, 4H).
[0082] (b) (2Z)-4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy ]-Sodium 4-oxo-2-butenoate.
[0083]
[0084] Take 21.3 g (0.05 mol) of the product from the previous step, dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com