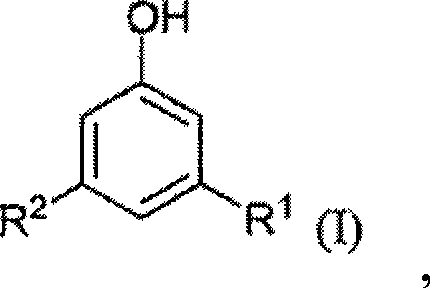
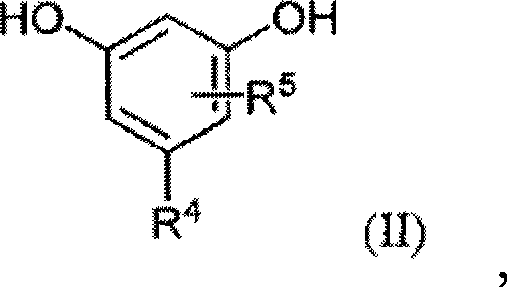
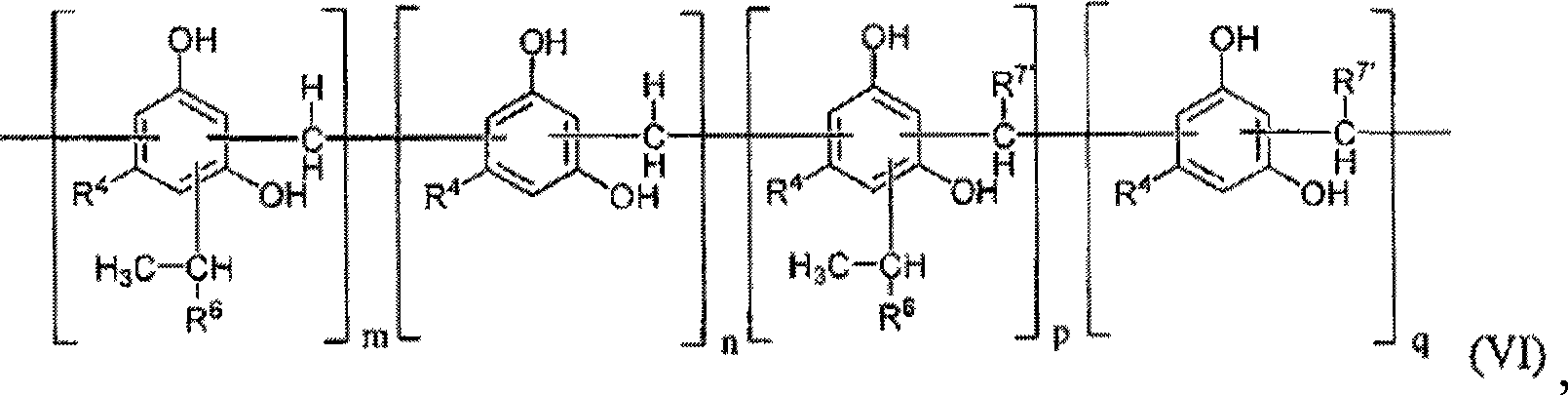
Modified alkylresorcinol resins and applications thereof
A technology of alkyl resorcinol resin, alkyl resorcinol, applied in the application field of rubber composition formulation, can solve problems such as health and environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Synthesis of Alkyl Resorcinol-Formaldehyde and Alkyl Resorcinol-Resorcinol-Formaldehyde Resins
[0103] Table 2. Synthesis of Alkyl Resorcinol-Formaldehyde Resins
[0104]
Resin number
1 2 3 4 5 6 7 8 Raw material (mole)
Resorcinol
Resorcinol homopolymer
HONEYOL
Resin properties
Softening point (℃)
Free resorcinol (% by weight)
Free 2-methylresorcinol
(%weight)
Free 5-methylresorcinol
(%weight)
Free 2,5-dimethylm-benzene
Diphenol (% by weight)
0 0 0 0.18 0.36 0.54 1.20 0
0 0 0 0 0 0 0 0.75
1.67 1.67 1.67 1.50 1.30 1.17 0.67 0.88
0.88 0.79 0.84 0.84 0.85 0.86 0.83 1.01
112.9 96.3 103.3 103.1 100.7 98.7 83.6 115.5
0.6 1.1 0.5 2.7 5.0 7.5 17.0 5.6
<0.1 <0.1 0.51 0.38 0.53 0.49 <0.05 <0.05
5.6 7.6 7.2 6.9 6.6 5.7 3.9 1.9
1.6 1.7 1.9 1.6 1.5 1.2 ...
Embodiment 2
[0111] Synthesis of Alkyl Resorcinol-Styrene-Formaldehyde Resin
[0112] Table 3. Synthesis of Alkyl Resorcinol-Styrene-Formaldehyde Resins
[0113]
Resin number
9 10 11 12 13 14 Raw material (mole)
HONEYOL
Resin properties
Softening point (℃)
Free resorcinol (% by weight)
Free 2-methylresorcinol
(%weight)
Free 5-methylresorcinol
(%weight)
Free 2,5-dimethylisophthalic di
Phenol (% by weight)
1.49 1.49 1.49 1.49 1.49 1.49
0.80 0.64 0.64 0.38 0.25 0.50
0.73 0.73 0.73 0.73 0.73 0.73
95.4 100.7 98.0 97.8 99.1 98.7
0.04 0.14 0.05 0.17 0.14 0.03
<0.05 <0.05 <0.01 <0.01 <0.01 <0.01
0.6 ...
Embodiment 3
[0122] Testing of Alkyl Resorcinol-Formaldehyde Resins
[0123] Rubber compounds B and C were prepared using alkylresorcinol-formaldehyde resins 1 and 2, respectively, according to the procedure described above, and the acceptor / donor ratios were as shown in Table 4 below. Use separately Resin B-19-S, VKG SF-281 and VKG AFES were used as methylene acceptors, and rubber compounds A, D and E were similarly prepared as controls. The physical properties of the rubber compounds were evaluated accordingly and the test results are listed in Table 4 below. The data in Table 4 show that Compounds A-E are similar in Mooney viscosity, rheological cure, wire adhesion, dynamic mechanical properties, Shore A hardness values, tensile properties, and Type C die tear properties.
[0124] Table 4.
[0125]
[0126]
[0127] Note: Resin B-19-S was obtained from INDSPEC Chemical Corporation, Pittsburgh, PA. VKG SF-281 and VKG AFES were obtained from VKG Oil AS, Kohtla-Jarve, Estonia....
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com