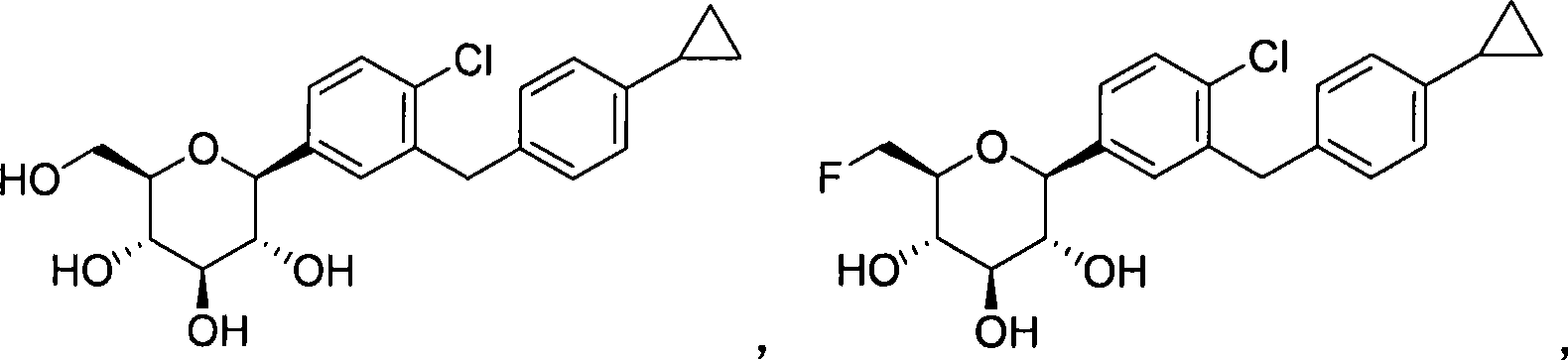
C-aryl glucoside SGLT2 inhibitor
An alkyl and cyano technology, which can be used in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., and can solve the problems of low activity and insufficient efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] Compound preparation
[0076] Those skilled in the art should understand that after knowing the structure of the compound of the present invention, the compound of the present invention can be obtained by various methods well known in the art and using known raw materials, such as chemical synthesis or extraction from plants, These methods are all included in the present invention. Unless otherwise stated or a method of preparation is provided, the starting materials used in the preparation of the compounds of the present invention or their intermediates are known in the art or are commercially available.
[0077] As a preferred mode of the present invention, the compound of the present invention can be prepared through the following reaction scheme and description.
[0078] Process 1
[0079]
[0080] As shown in Scheme 1, at -78°C, the compound of formula V is treated with n-butyllithium or tert-butyllithium in a mixed solvent of tetrahydrofuran / toluene, and then...
Embodiment 1
[0117] The preparation of embodiment 1 intermediate compound
[0118] 1. Preparation of (2-chloro-5-bromo-phenyl)-(phenyl)-methanone
[0119]
[0120] At room temperature, 41 mL (477 mmol) of oxalyl chloride and 0.64 mL of N,N-dimethylformamide were added to a solution of 100 g (425 mmol) of 2-chloro-5-bromobenzoic acid dissolved in 300 mL of dichloromethane. The mixed solution was stirred overnight at room temperature, and then all volatile components were distilled off under reduced pressure. The resulting solid residue was redissolved in 100 mL of dichloromethane, the solution was cooled to -5°C, and 76 mL (850 mmol) of benzene was added. Then 62.4 g (468 mmol) of aluminum trichloride were added in portions. The reaction mixture was stirred at -3°C for 10 minutes, then slowly warmed to room temperature, and stirring was continued at room temperature for 1 hour. The reaction mixture was poured into a beaker filled with ice, the organic phase was separated, and the aque...
Embodiment 2
[0196] The preparation of embodiment 2 final product
[0197] 1. Preparation of 1-chloro-2-(4-cyclopropylbenzyl)-4-(β-D-glucopyranose-1-yl)-benzene (compound A)
[0198]
[0199] 105 mg (0.18 mmol) of 1-chloro-2-(4-cyclopropylbenzyl)-4-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranose A solution of -1-yl)-benzene and 4.7 mg (0.11 mmol) of lithium hydroxide monohydrate in 1 mL of 2:3:1 THF / methanol / water was stirred at room temperature for 2 h, then concentrated, and the resulting residue was analyzed by preparative HPLC purification.
[0200] Obtained product: 38 mg (51% of theoretical yield).
[0201] 1 H NMR (400MHz, CDCl3) δ 7.33(d, J=8.0Hz, 1H), 7.31(d, J=2.0Hz, 1H), 7.26(dd, J=8.4, 2.4Hz, 1H), 7.05(d, J=8.0Hz, 2H), 6.94(d, J=8.0Hz, 2H), 4.08(d, J=9.2Hz, 1H), 4.06(d, J=14.8Hz, 1H), 4.00(d, J= 14.8Hz, 1H), 3.86(dd, J=12.0, 1.6Hz, 1H), 3.68(dd, J=11.6, 5.2Hz, 1H), 3.47-3.37(m, 3H), 3.28(t, J=8.4 Hz, 1H), 1.85-1.80(m, 1H), 0.92-0.87(m, 2H), 0.62-0.58(m, 2H);
[0202] LC-MS(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com