Modulators of metabolism and the treatment of disorders related thereto
A related disease, effective dose technology, applied in the direction of metabolic diseases, medical preparations containing active ingredients, diseases, etc., can solve the problems of increased risk of death, failure to consider the proportion of muscle constitution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
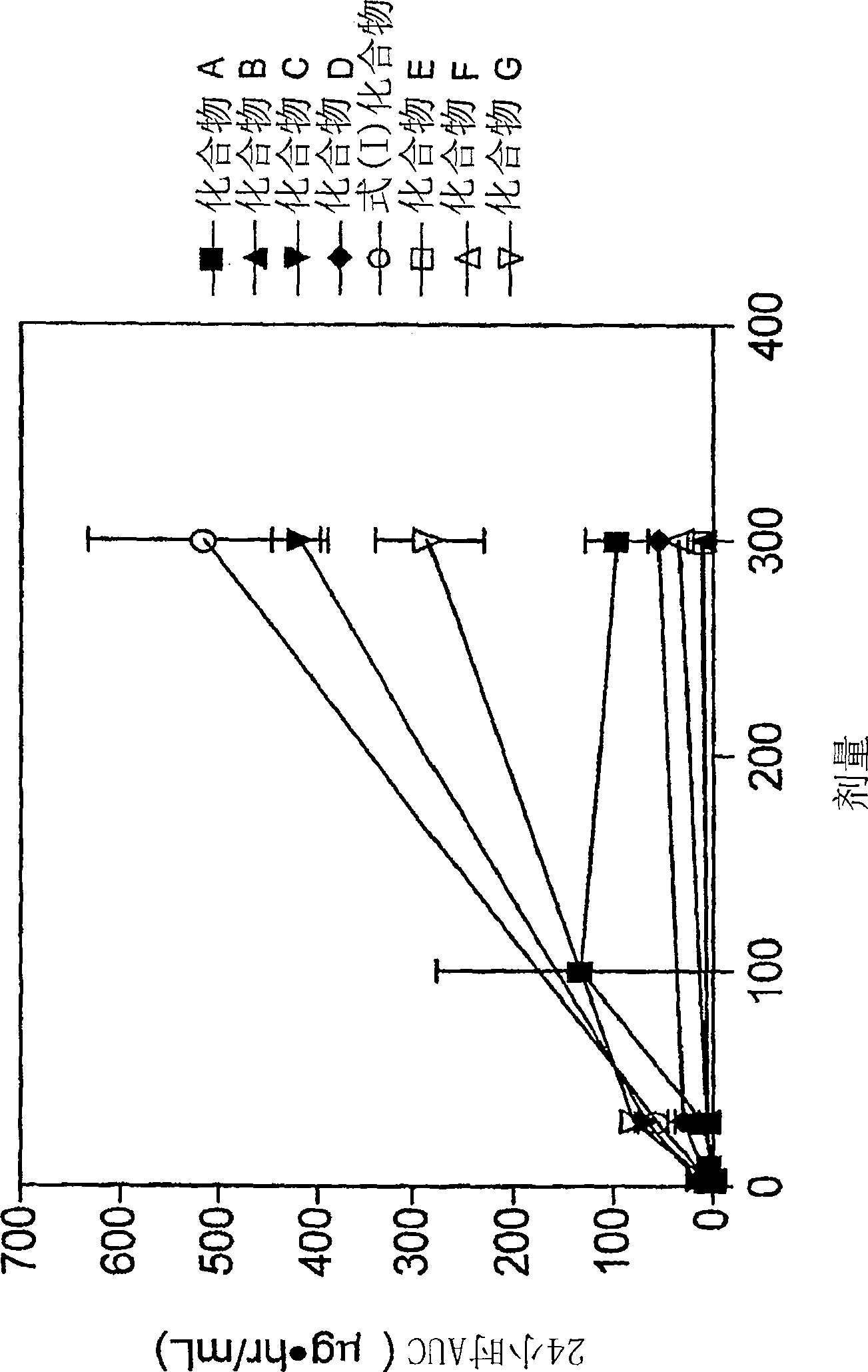
[0138] Example 1: In vivo effects of RUP3 agonists on glucose homeostasis in rats
[0139] General Procedure-Oral Glucose Tolerance Test (oGTT)
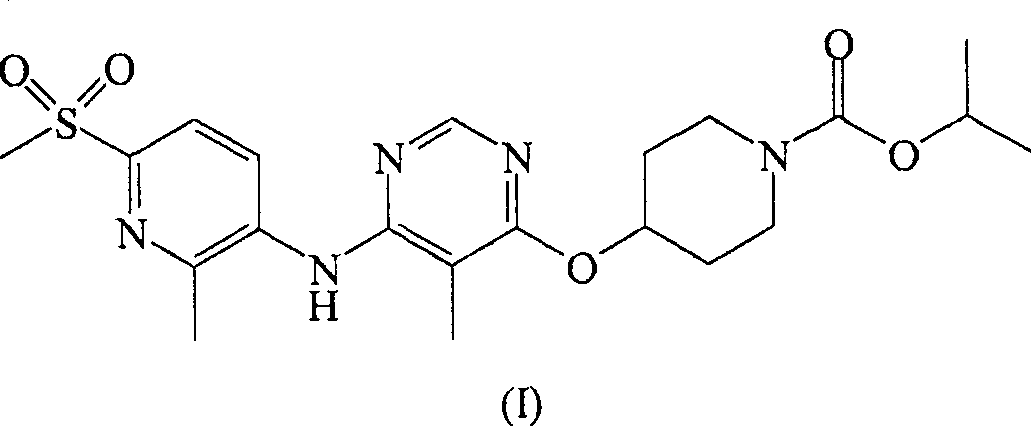
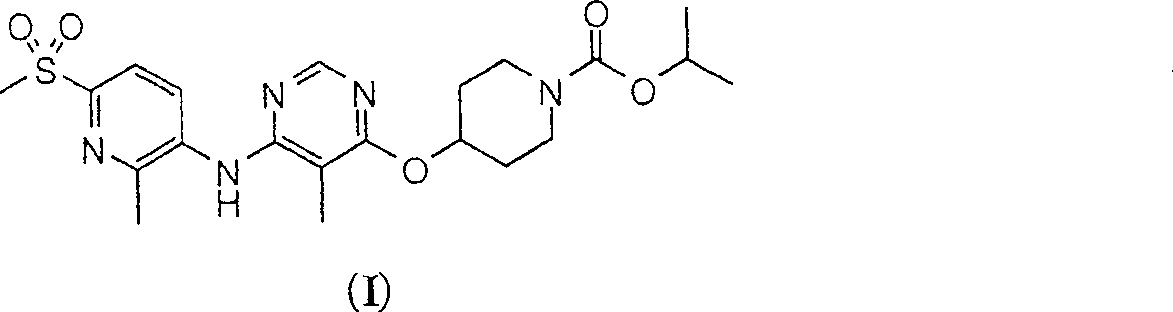
[0140] Male Sprague Dawley rats (Harlan, San Diego, California (CA)) weighing about 350-375g were fasted for 16 hours and randomly divided into groups (n=6) To receive 0.3mg / kg, 3mg / kg or 30mg / kg RUP3 agonist. The compound was delivered orally via a gavage needle (oral, volume 2 mL / kg). At the beginning of the test, a blood glucose meter (Accu-Chek Advantage, Roche Diagnostics) was used to assess blood glucose levels, and a vehicle (20% hydroxypropyl-β-cyclodextrin) was administered to rats Or test compound. Thirty minutes after the test compound was administered, the blood glucose level was evaluated again, and the rats were orally administered with 3 g / kg dextrose. Then blood glucose measurements were taken 30 minutes, 60 minutes, and 120 minutes after this time. RUP3 agonist 4-[6-(6-methanesulfonyl-2-methyl-pyridin-3-ylamino)-5-methyl...
example 2
[0141] Example 2: Receptor binding assay
[0142] In addition to the methods described herein, another method of evaluating test compounds is by determining their binding affinity to the RUP3 receptor. This type of assay usually requires a radiolabeled ligand for the RUP3 receptor. Without using the known ligands of the RUP3 receptor and its radiolabel, the compound of formula (I) can be labeled with a radioisotope and used in the assay to evaluate the affinity of the test compound with the RUP3 receptor.
[0143] The radiolabeled RUP3 compound of formula (I) can be used in screening assays to identify / evaluate compounds. In general, the ability of newly synthesized or newly identified compounds (ie, test compounds) to reduce the ability of the "radiolabeled compound of formula (I)" to bind to the RUP3 receptor can be evaluated. Therefore, the ability of the test compound to compete with the "radiolabeled compound of formula (I)" or the radiolabeled RUP3 ligand for binding to the ...
example 3
[0155] The following examples further illustrate the compounds of the present invention and their synthesis. The following examples are provided to further illustrate the present invention, without restricting the present invention to the details of these examples. The compounds described in this article are named according to CS ChemDrawUltra version 7.0.1. In some cases, common names are used and those skilled in the art should recognize these common names.
[0156] Chemistry: Proton Nuclear Magnetic Resonance ( 1 H NMR spectrum is on Varian Mercury Vx-400 equipped with 4-core automatic switchable probe and z-gradient field or equipped with QNP (Quad Nucleus Probe) or BBI (Broad Band Inverse) probe and z-gradient field on Bruker Avance-400 (Bruker Avance-400). The chemical shift is expressed in parts per million (ppm), where the residual solvent signal is used as a reference. NMR abbreviations are used as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com