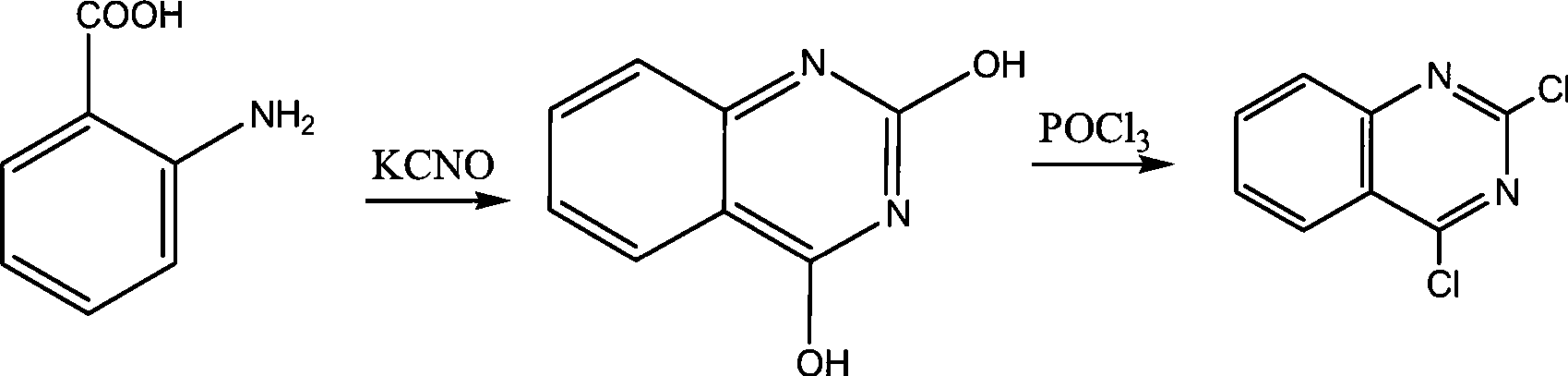
Preparation of 2,4-dichloroquinazoline
A technology of dichloroquinazoline and quinazoline dione, applied in 2 fields, can solve the problems of high production cost, low reaction yield, unsuitable for industrialized production and the like, and achieves a technology with low production cost, low solvent toxicity and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of 2,4-dichloroquinazoline, comprises the steps successively:
[0024] (1), anthranilic acid reacts with potassium cyanate to generate 2,4-quinazolinedione
[0025] Take a dry 500ml four-neck flask, install a thermometer and a cooling tube, add 41g (0.3mol) anthranilic acid and 100ml water to form a mixture, then add dropwise 200ml aqueous solution of 55g (0.68mol) potassium cyanate, and stir for half an hour Add 36g of 0.5mol / L sodium hydroxide aqueous solution dropwise, control the temperature of the solution within 40°C, stir for half an hour, heat up to 85°C, keep it warm for 2 hours, cool to 0°C, add 30% hydrochloric acid to acidify, and adjust the pH to 2~3 , the temperature was kept at about 0° C., filtered and dried to obtain 42 g of 2,4-quinazolinedione, and the molar yield was 86.3% based on 2,4-quinazolinedione.
[0026] (2), 2,4-quinazoline diketone chlorination obtains 2,4-dichloroquinazoline
[0027] Take a 500ml dry four-nec...
Embodiment 2
[0029] A kind of preparation method of 2,4-dichloroquinazoline, comprises the steps successively:
[0030] (1), anthranilic acid reacts with potassium cyanate to generate 2,4-quinazolinedione
[0031] Take a dry 500ml four-neck flask, install a thermometer and a cooling tube, add 41g (0.3mol) anthranilic acid and 100ml water to form a mixture, then add dropwise 200ml aqueous solution of 60g (0.74mol) potassium cyanate, and stir for half an hour Add 60g of 0.3mol / L sodium hydroxide aqueous solution dropwise, control the temperature of the solution within 40°C, stir for half an hour, heat up to 90°C, keep it warm for 2.5 hours, cool to 0°C, add 30% hydrochloric acid to acidify, and adjust the pH to 2~3 , the temperature was kept at about 0° C., filtered and dried to obtain 45 g of 2,4-quinazolinedione, and the molar yield was 92.5% based on 2,4-quinazolinedione.
[0032] (2), 2,4-quinazoline diketone chlorination obtains 2,4-dichloroquinazoline
[0033] Get a 500ml dry four-ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com