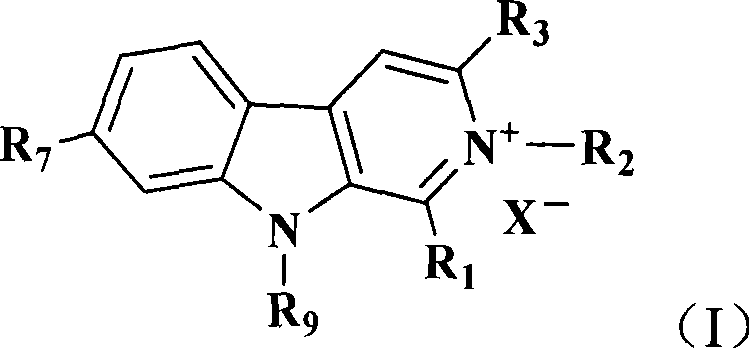
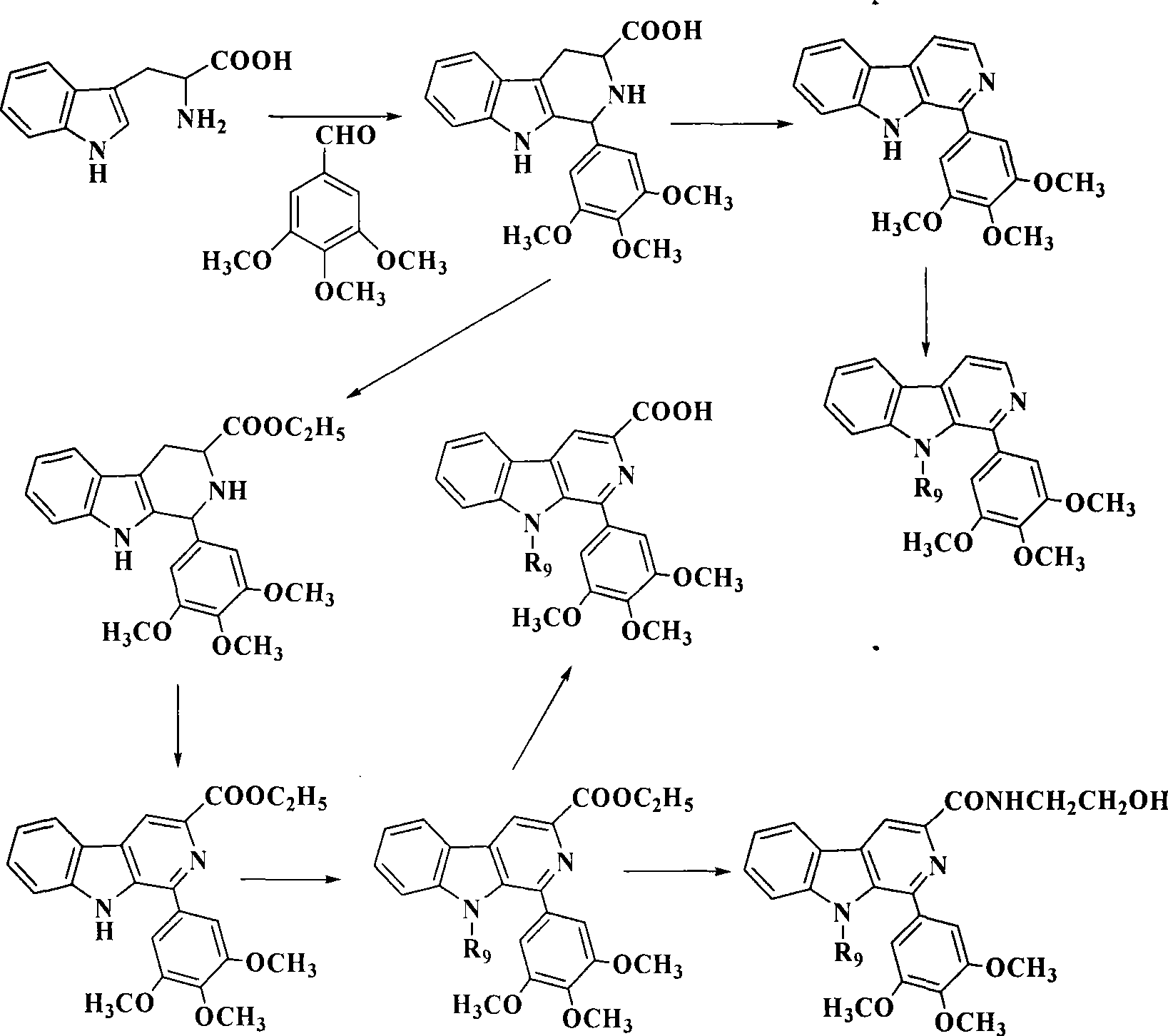
Banisterine derivant and uses thereof
A technology of dehydrohalampine and its derivatives, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as high neurotoxicity, high tumor inhibition rate, and no clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
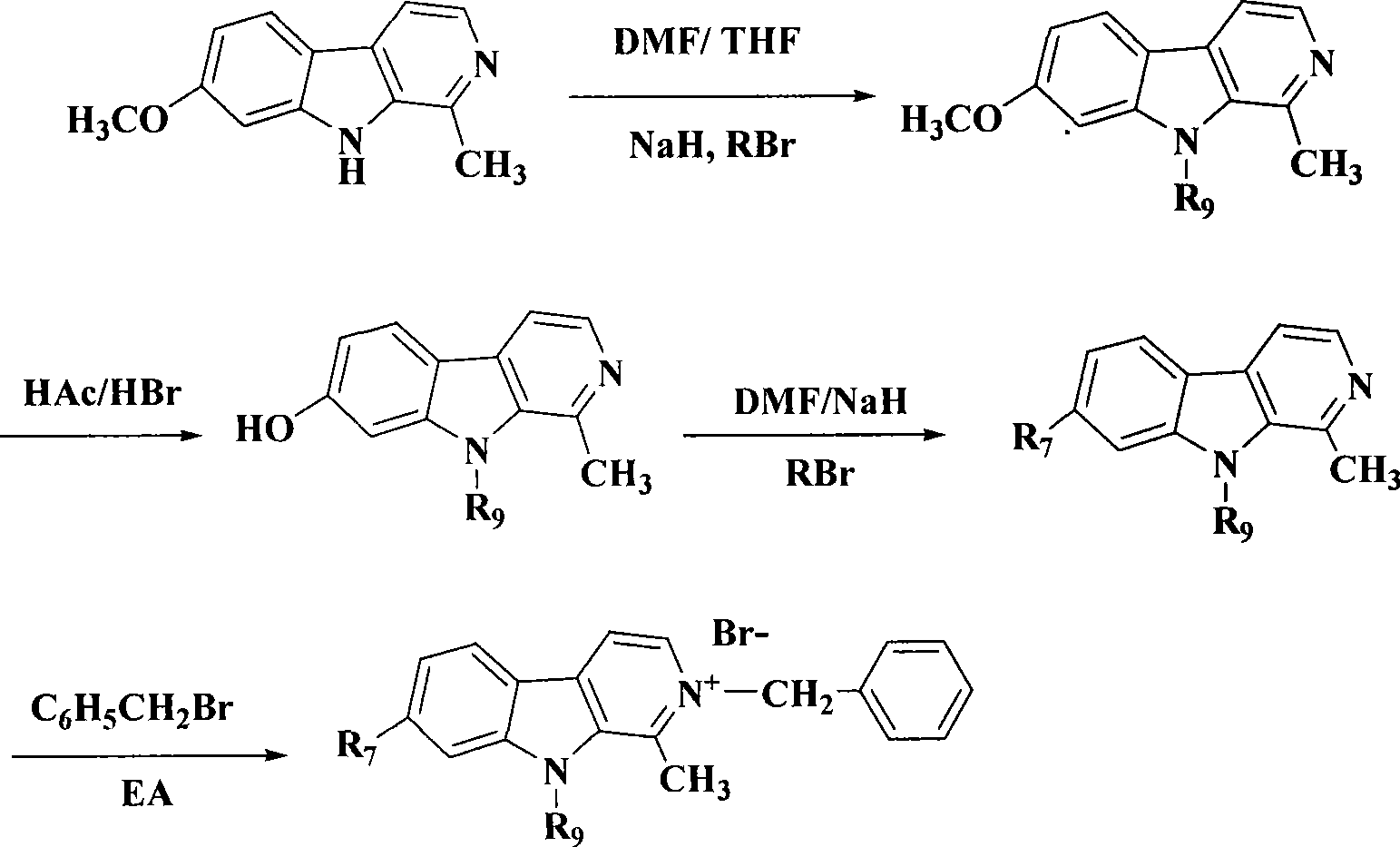
[0323] Example 1 7-Hydroxy-9-ethyl-1-methyl-β-carboline (1)
[0324] Step A: 7-Methoxy-9-ethyl-1-methyl-β-carboline
[0325] Harmline (2.12g, 10mmol), DMF (50ml), 60% NaH (0.6g, 15mmol) were mixed, stirred at room temperature for 10min, added ethyl iodide (15mmol), stirred at room temperature, followed by TLC detection. After the reaction was completed, the reaction mixture solution was poured into 100 ml of ice water, stirred at room temperature for 2 hours, filtered, and washed with a large amount of water to obtain a light yellow solid. Dissolve the above solid in absolute ethanol, adjust the pH to 2 with concentrated hydrochloric acid, then concentrate under reduced pressure to dryness, add water to the absolute ethanol three times to obtain a yellow oil, recrystallize from acetone, and filter to obtain white crystals. Hydrochloride. It was directly used in the next reaction without further purification.
[0326] Step B: 7-Hydroxy-9-ethyl-1-methyl-β-carboline
[0327] ...
Embodiment 2
[0331] Example 2 7-hydroxyl-9-n-butyl-1-methyl-β-carboline (2)
[0332] The operation is the same as in Example 1, but in step A, n-butane iodide is used as the alkylating agent. 2.2 g of white crystals were obtained, yield 87%, mp 205-206°C;
[0333] FAB-MS m / e(M+1)255;
[0334] IR (KBr) 3500-1750, 1618, 1566, 1492, 1451, 1413, 1325, 1238, 1187, 1137, 1112, 980;
[0335] 1 H-NMR (500MHz, DMSO-d 6 )δ 9.72 (1H, s, OH); 8.12-8.13 (1H, d, J=5.0Hz, H-3); 7.95-7.97 (1H, d, J=8.5Hz, H-4); 7.78-7.79 (1H, d, J=5.0Hz, H-5); 6.90-6.91 (1H, m, H-6); 6.72-6.75 (1H, m, H-8); 4.41-4.44 (2H, m, NCH 2 CH 2 CH 2 CH 3 ); 2.91 (3H, s, CH 3 ); 1.67-1.74 (2H, m, NCH 2 CH 2 CH2 CH 3 ); 1.36-1.40 (2H, m, NCH 2 CH 2 CH 2 CH 3 ); 0.92-0.94 (2H, m, NCH 2 CH 2 CH 2 CH 3 ).
Embodiment 3
[0336] Example 3 7-Hydroxy-9-isobutyl-1-methyl-β-carboline (3)
[0337] The operation was the same as in Example 1, except that 1-iodo-2-methylpropane was used as the alkylating agent in step A. 2.0 g of white crystals were obtained, yield 79%, mp 246-248°C;
[0338] FAB-MS m / e(M+1)255;
[0339] IR (KBr) 3500-1750, 1626, 1568, 1447, 1392, 1203, 1136, 977, 820;
[0340] 1 H-NMR (500MHz, DMSO-d 6 )δ 9.70 (1H, s, OH); 8.13-8.14 (1H, d, H-3); 7.95-7.97 (1H, d, H-4); 7.79-7.80 (1H, d, H-5); 6.93 (1H, s, H-6); 6.72-6.74 (1H, d, H-8), 4.26-4.27 (2H, d, NCH 2 CH[CH 3 ] 2 ); 2.90 (3H, s, CH 3 ); 2.11-2.17 (1H, m, NCH 2 CH[CH 3 ] 2 ); 0.85-0.87 (6H, s, NCH 2 CH[CH 3 ] 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com