Dried biotherapeutic composition, uses, and device and methods for administration thereof
A technology of biological therapy and composition, which is applied in the direction of drug combination, medical packaging, medical preparations containing active ingredients, etc., and can solve problems such as adverse gastrointestinal side effects and high blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1: the method for preparing bacterium
[0109] Bacteria of choice are first prepared for growth to obtain a concentration range in 0.3%-0.6% NaCl solution10 11 -10 12 CFU / ml of biomass to generate autolysates.
[0110] liquid medium
[0111] For the preparation of bacterial biomass, standard fermentation vessels with aeration can be used. Nutrients necessary for bacterial growth are added in two stages: in the first stage, as part of the initial batch medium, and in the second stage, as a continuous supplemental feed after the nutrients have been depleted in the production reactor liquid.
[0112] During a typical fermentation, the medium may consist of a suitable nitrogen source, glucose, sodium chloride, and a combination of disodium hydrogen phosphate and potassium dihydrogen phosphate sufficient to provide a neutral or slightly alkaline pH (7.2 ± 0.2).
[0113] Exemplary media contain phosphates, such as sodium and potassium phosphate; magnesium sulf...
Embodiment 2
[0131] Example 2: Preparation of Biotherapeutic Compositions - Exemplary Methods
[0132] Compositions according to the invention may optionally be prepared according to the following exemplified methods. Using standard microbial fermentation techniques, with optional probiotic Escherichia coli (10 8 -10 9 cells) to inoculate liquid or solid media components. Growth conditions preferably include continuous aeration, maintenance of neutral pH, and glucose supplementation. Such organisms are preferably not genetically engineered in any way, but are isolated from microflora obtained from the gastrointestinal tract of a normal human.
[0133] Manufacturing is optionally and preferably controlled with respect to the following critical control points:
[0134] Carefully receive and handle cultures;
[0135] Controlling processes to ensure proper culture conditions;
[0136] maintain sterility;
[0137] Processes are controlled to ensure the correct level of probiotics in the ...
Embodiment 3
[0146] Example 3: Exemplary Applicator
[0147] This example describes a number of different non-limiting, exemplary device embodiments for storing and administering biotherapeutic compositions according to the invention.
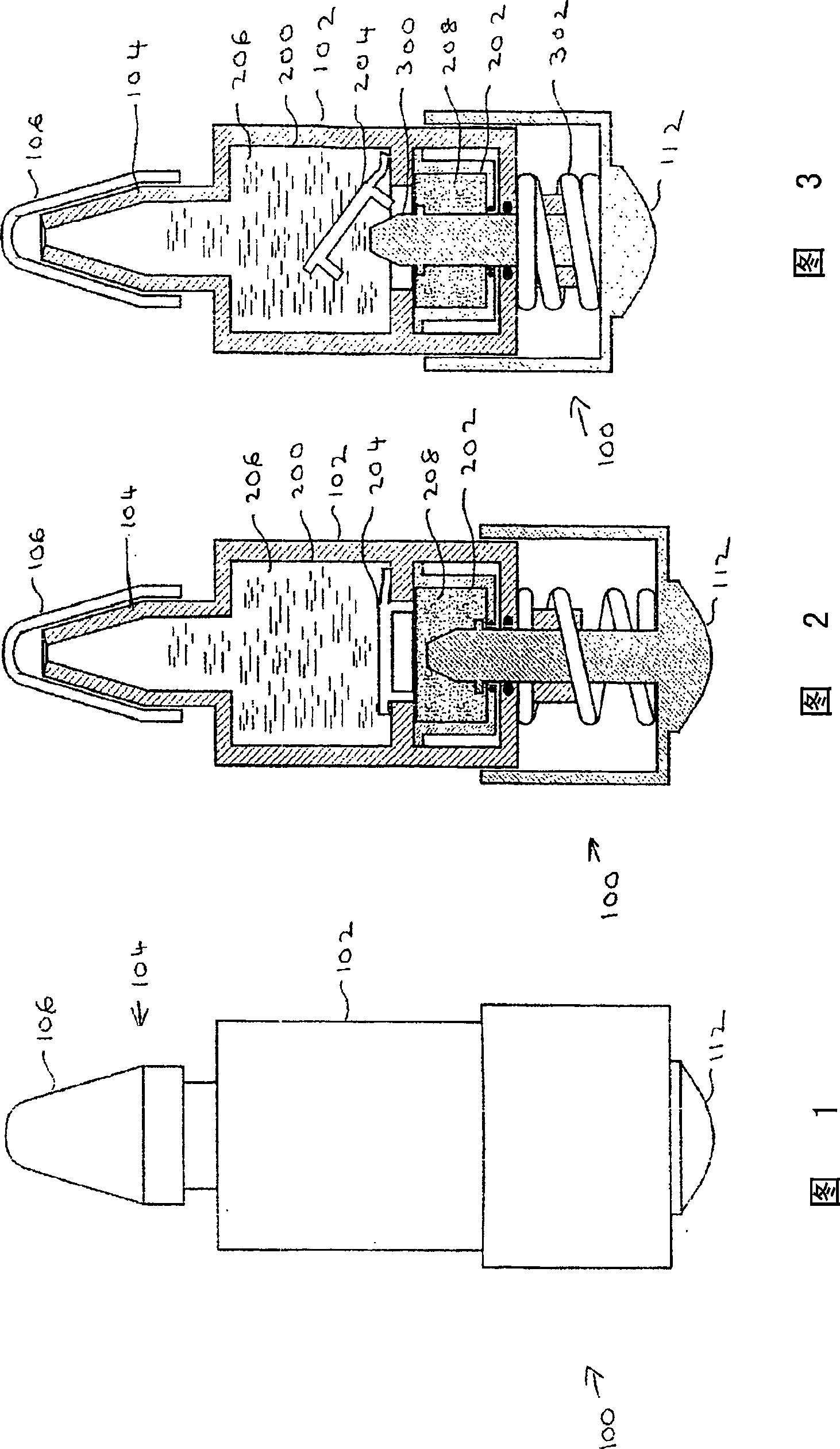
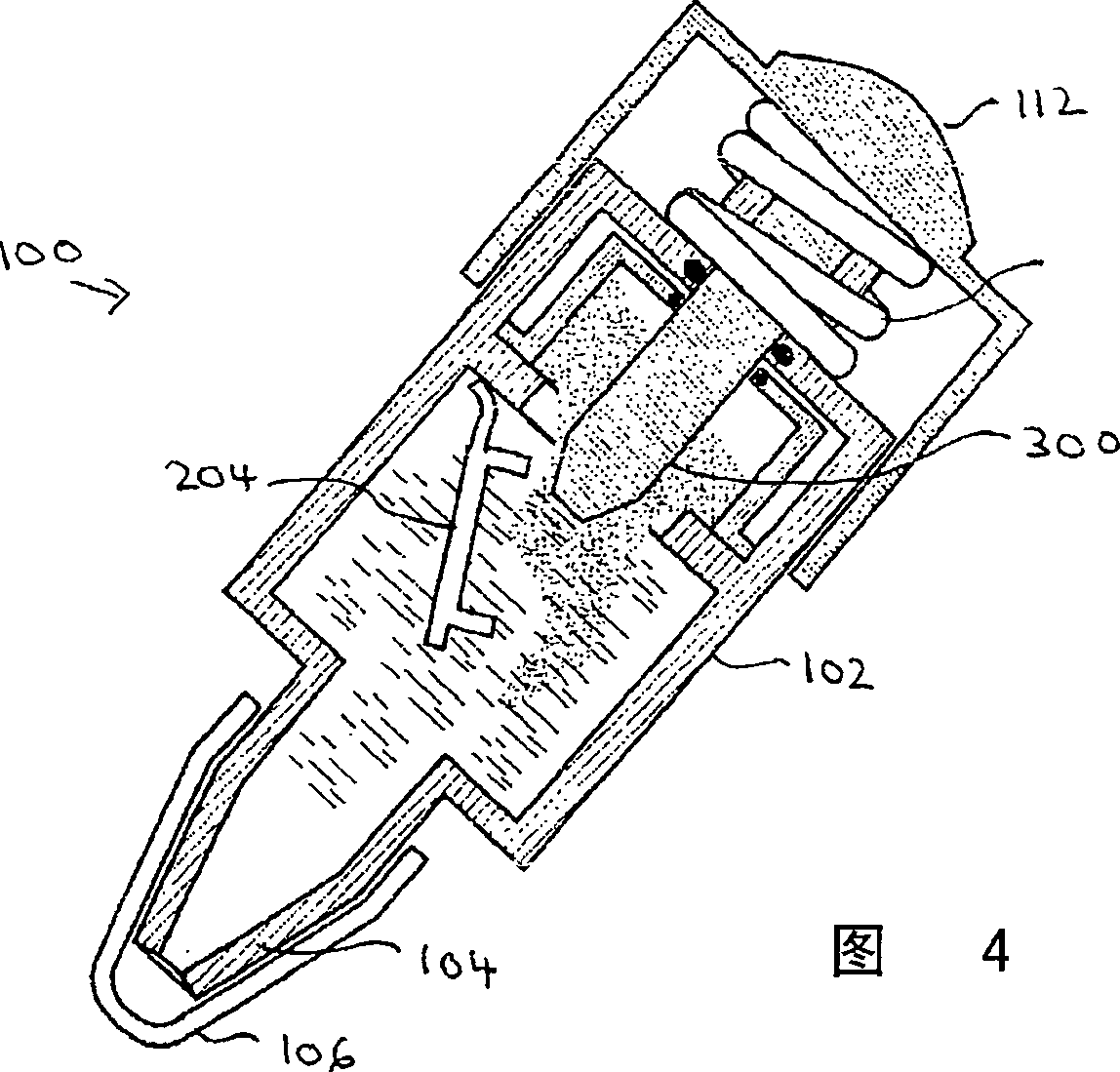
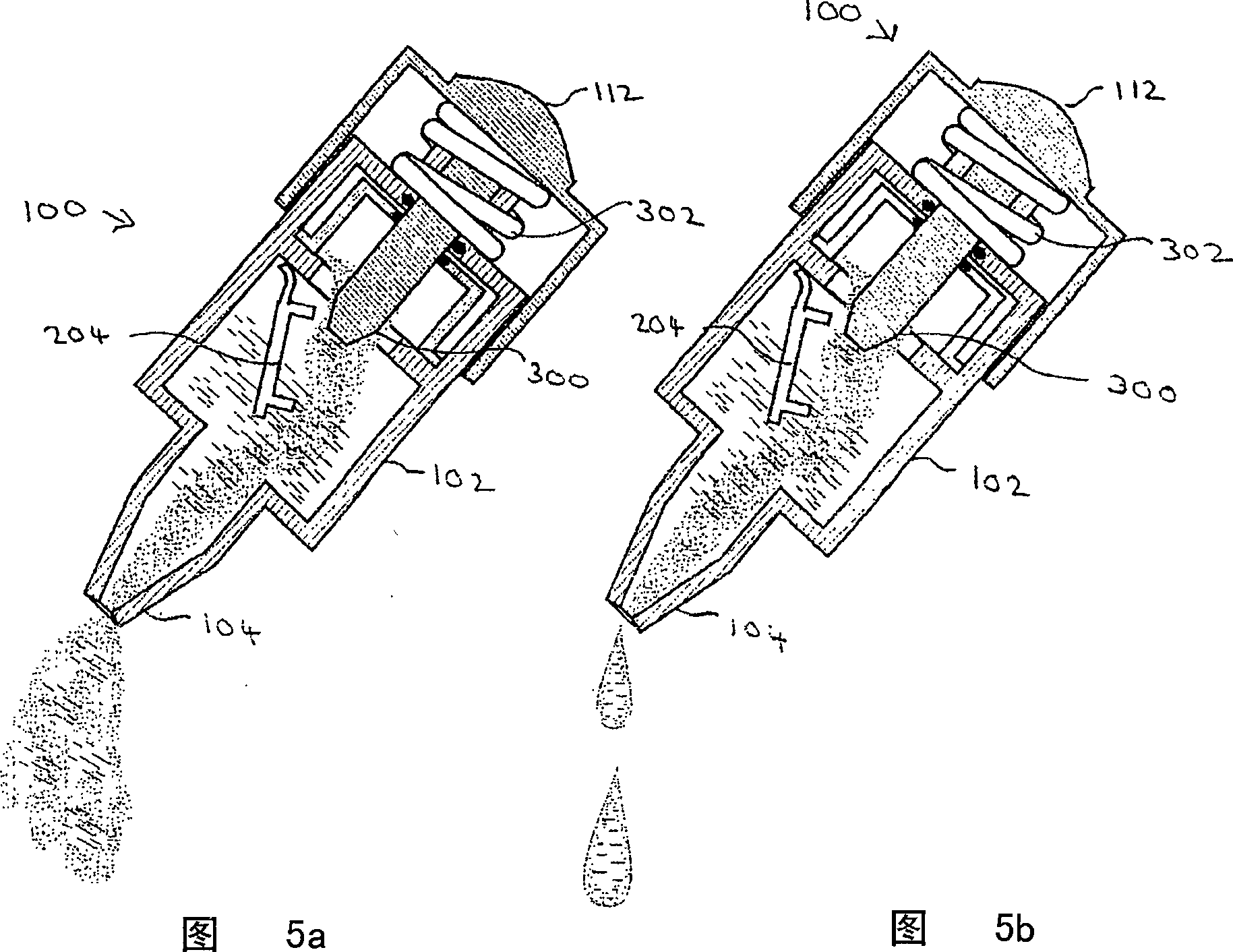
[0148] As shown in Figure 1, an exemplary device 100 in accordance with the present invention features a body 102 having a plurality of compartments (not shown, see Figure 2). Body 102 preferably communicates with a nozzle 104 for administering the mixture to a subject. The nozzle 104 is preferably covered with a cap 106, which can be removed for administration.
[0149] The main body 102 is preferably divided into a plurality of sections, the function of which is described in more detail below with reference to FIGS. 2 and 3 . The device 100 also preferably provides a handle 112, the function of which is described in more detail below also with reference to FIGS. 2 and 3 .
[0150] 2 and 3 show device 100 in cross-section. In FIG. 2 , the display devic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com