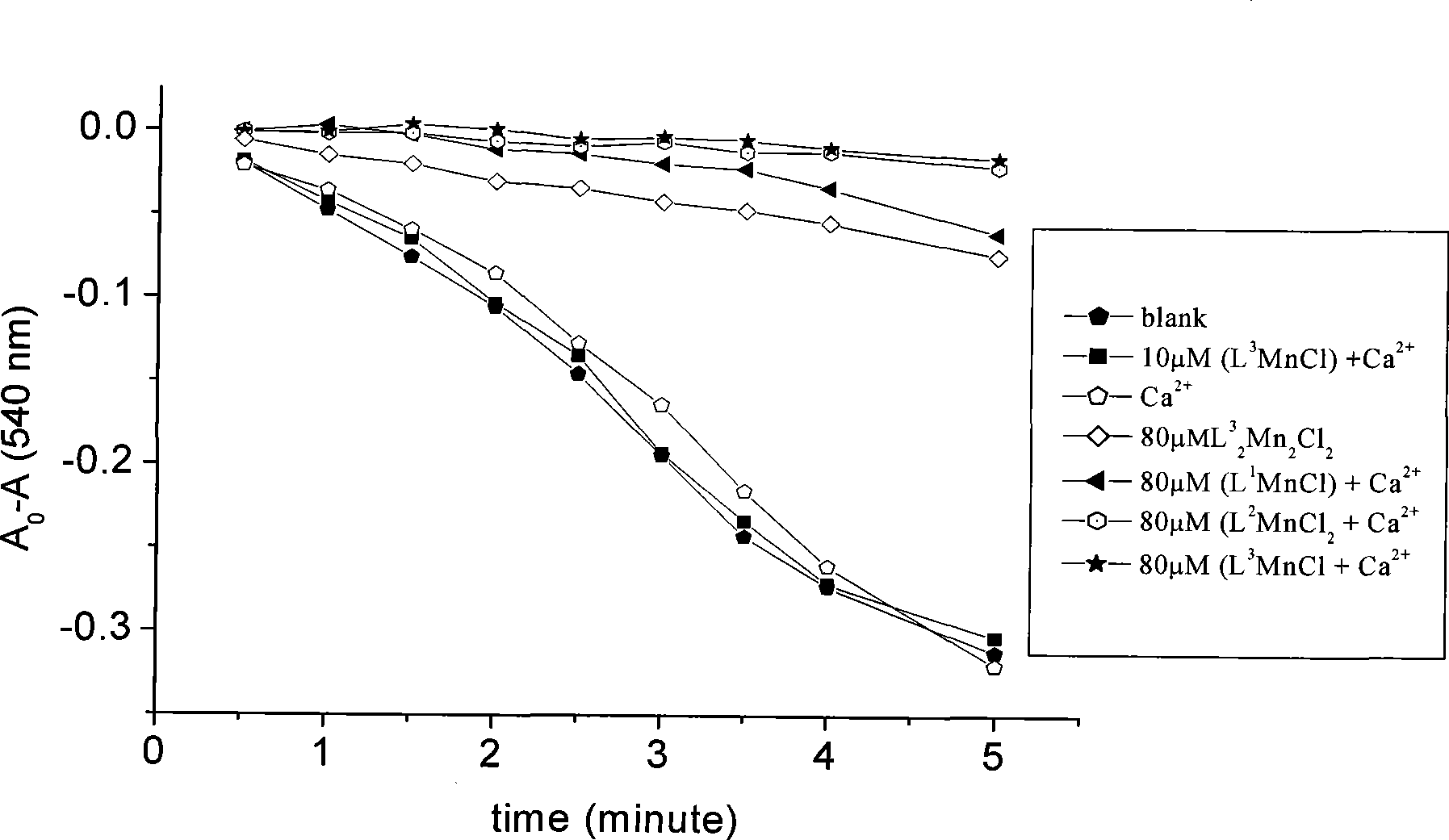
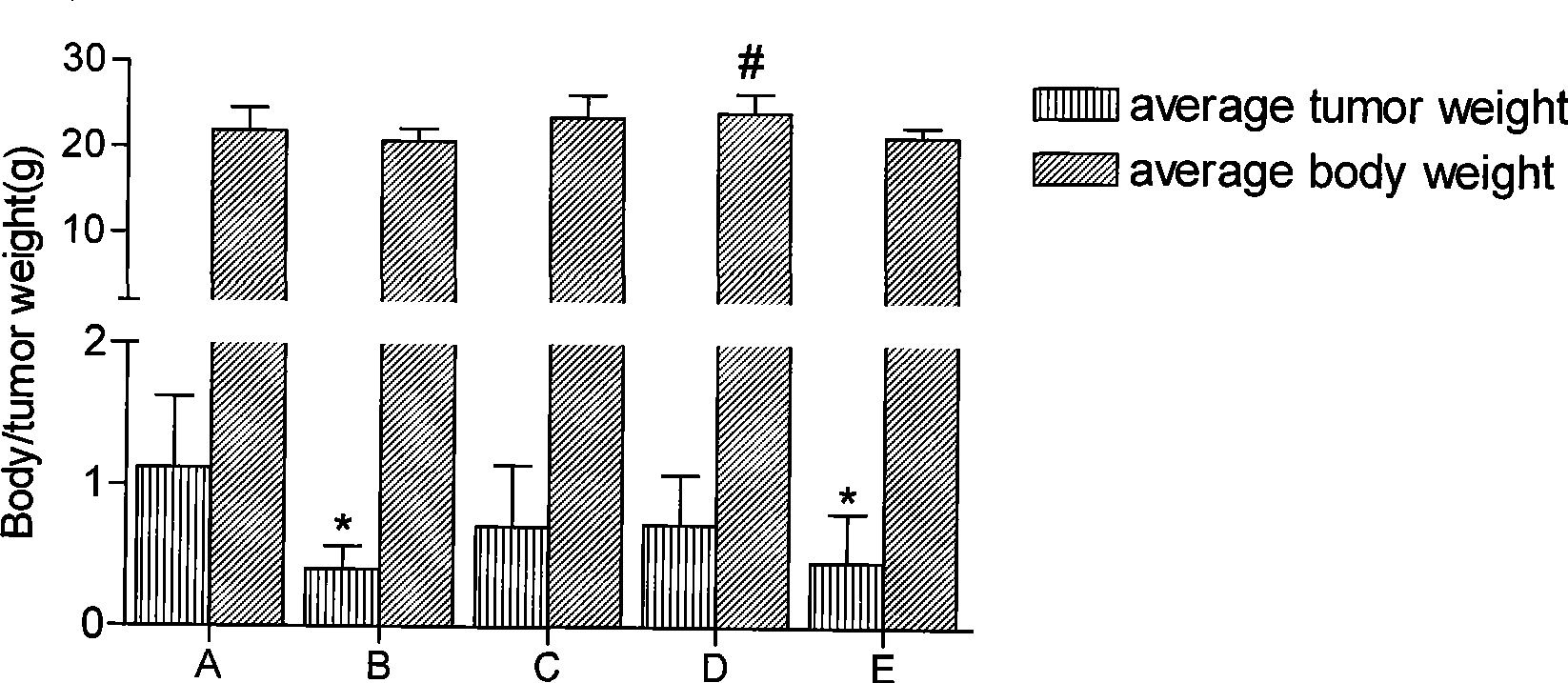
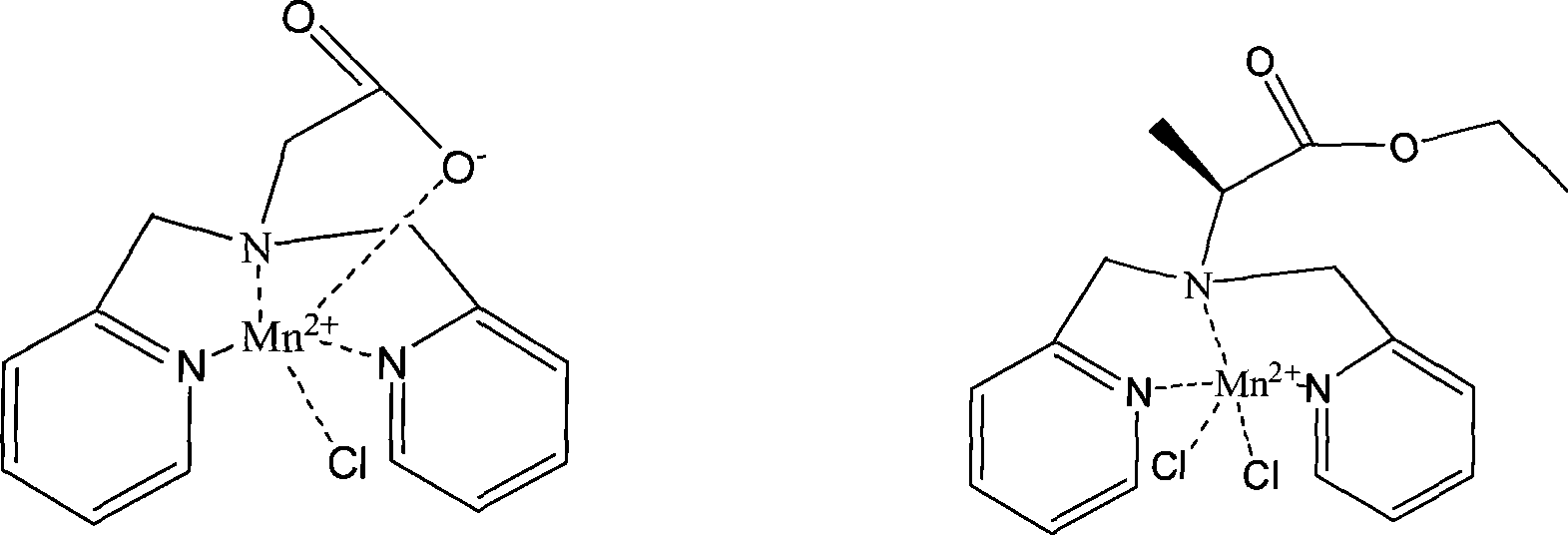
Anti-tumor bipyridine methyl substituted manganese amino acid complex, and preparation and application thereof
A manganese complex and picolinyl amine group technology are applied in the field of targeted anti-tumor dipyridylmethyl substituted amino acid manganese complexes and their preparation, achieving the effect of strong recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 (best reaction condition example):
[0038] The ligand bis(2-pyridylamino)acetic acid (L 1 ) and MnCl 2 Dissolve in aqueous solution at a molar ratio of 1:1, control the reaction temperature at 50°C, and the reaction time for 3 hours, remove the solution to obtain a yellow manganese complex: [L 1 MnCl] or . Yield: 80%. Molecular formula: C 14 h 14 ClN 3 o 2 Mn. Elemental analysis: found C, 48.78%; H, 4.03%; N, 12.09; Mn, 15.92%; calculated C, 48.50; H, 4.07; N, 12.12; Infrared spectral data (IR, cm-1): 2936, 1624, 1572, 1493, 1035, 841, 776, 506.
[0039] Ligand L-bis(2-pyridylmethylamino) ethyl propionate) (L 2 ) and MnCl 2 Soluble in aqueous solution at a molar ratio of 1:1, the optimal reaction temperature is 50°C, and the optimal reaction time is 3 hours. Go to the solution to obtain a yellow manganese complex: [L 2 MnCl 2 ]or. Yield: 82%. Molecular formula: C 17 h 21 Cl 2 N 3 o 2 Mn. Elemental analysis: found C, 48.18%; H, 4.93%; N, ...
Embodiment 2
[0043] The ligand bis(2-pyridylamino)acetic acid (L 1 ) and MnCl 2 Dissolve in aqueous solution at a molar ratio of 1:1.3, control the reaction temperature to 80°C, and the reaction time to 6 hours, remove the solution to obtain a yellow manganese complex: [L 1 MnCl] or . Yield: 74%. Molecular formula: C 14 h 14 ClN 3 o 2 Mn.
[0044] The ligand bis(2-pyridylamino)propionic acid (L 2 ) and MnCl 2 Dissolve in methanol solution at a molar ratio of 1:1.2, add 0.45 g of NaOH, control the reaction temperature to 78°C, and the reaction time is 8 hours, evaporate the methanol to obtain the yellow manganese complex: [L 2 MnCl]. Yield: 65%. Molecular formula: C 17 h 21 Cl 2 N 3 o 2 Mn.
[0045] The ligand L-bis(2-pyridylaminopropionic acid) (L 3 ) and MnCl 2 Dissolve in methanol solution at a molar ratio of 1:1.2, add 0.45 g of NaOH, control the reaction temperature to 68°C, and the reaction time is 6 hours, evaporate the methanol to obtain the yellow manganese complex...
Embodiment 3
[0048] The ligand bis(2-pyridylamino)acetic acid (L 1 ) and MnCl 2 Dissolve in aqueous solution at a molar ratio of 1:1.1, control the reaction temperature to 30°C, and the reaction time to 2 hours, remove the solution to obtain a yellow manganese complex: [L 1 MnCl] or . Yield: 44%. Molecular formula: C 14 h 14 ClN 3 o 2 Mn.
[0049] The ligand bis(2-pyridylamino)propionic acid (L 2 ) and MnCl 2 Dissolve in methanol solution at a molar ratio of 1:1.2, add 0.15 g of NaOH, control the reaction temperature to 30°C, and the reaction time is 8 hours, evaporate the methanol to obtain a light yellow manganese complex: [L 2 MnCl]. Yield: 45%. Molecular formula: C 17 h 21 Cl 2 N 3 o 2 Mn.
[0050] The ligand L-bis(2-pyridylaminopropionic acid) (L 3 ) and MnCl 2 Dissolve in methanol solution at a molar ratio of 1:1.1, add 0.25 g of NaOH, control the reaction temperature to 38°C, and the reaction time is 3 hours, evaporate the methanol to obtain a light yellow manganese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com