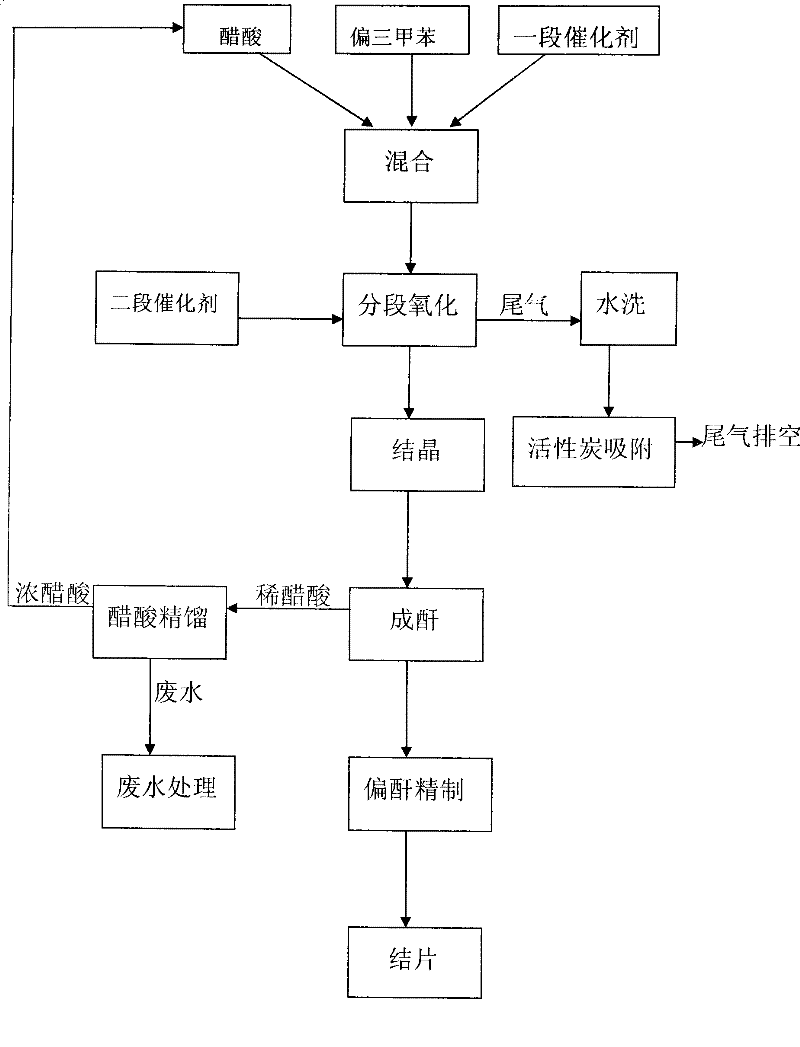
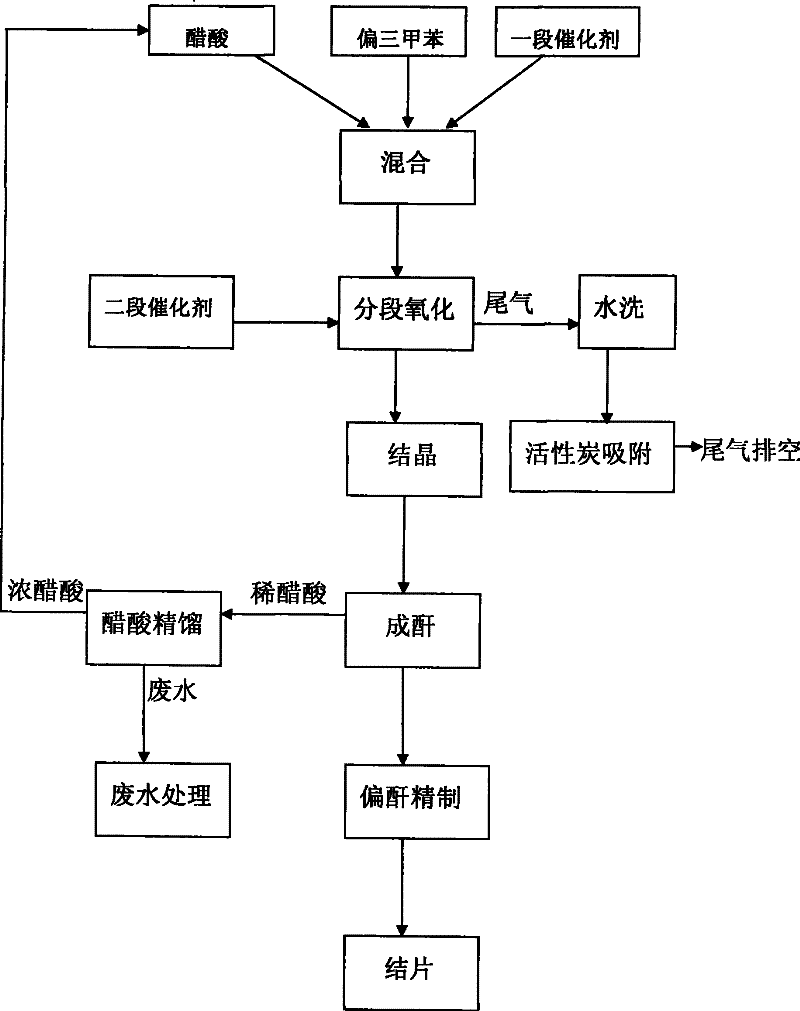
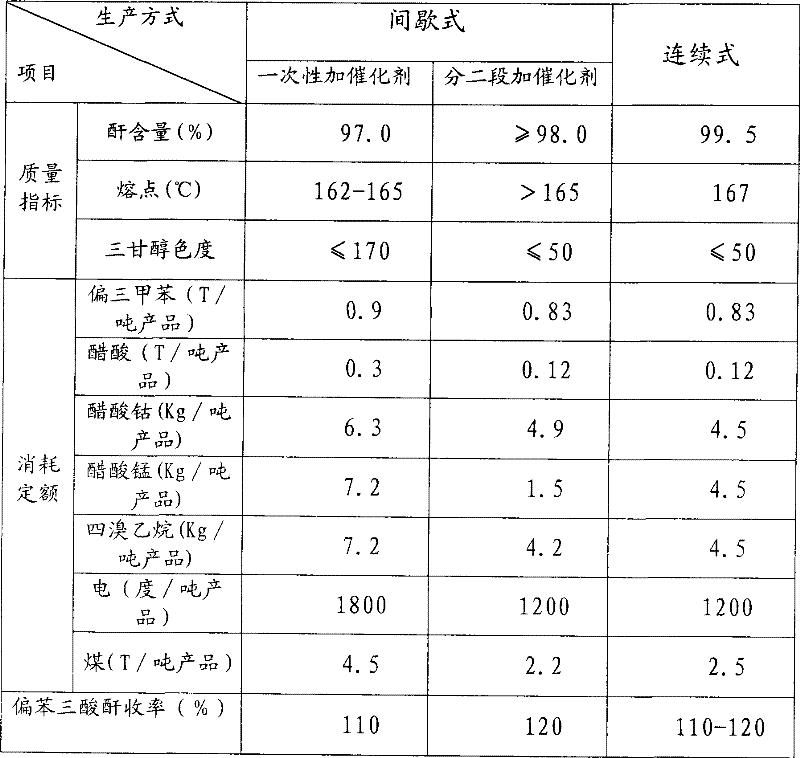
Method for producing trimellitic anhydride with pseudocumene liquid phase air segmenting hydrocarbonylation
A technology of trimellitic anhydride and trimethylbenzene, applied in the direction of organic chemistry, can solve the problems of low air utilization rate, inability to realize continuous operation optimization, air waste, etc., and achieve the effect of solving self-inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 partial anhydride
[0021] The main production process steps are as follows:
[0022] 1. Put 400Kg of mesitylene and 1000Kg of acetic acid into the mixing kettle, add 1.2Kg of cobalt acetate and 1.2Kg of tetrabromoethane. Control the mixing temperature to 60°C, start stirring and mixing to completely dissolve the catalyst.
[0023] 2. Put the mixture into the oxidation tower, raise the temperature to 140°C and the pressure to 1.0Mpa, introduce air to initiate a stage of oxidation reaction, control the oxidation temperature to 140-180°C, and the oxidation pressure to 0.4-1.0Mpa. After initiating for 30 minutes, put into the prepared catalyst, including 0.8Kg of cobalt acetate, 0.8Kg of tetrabromoethane, and 0.8Kg of manganese acetate. Control oxidation temperature 180-300℃, pressure 1.0-3.0Mpa, adjust air flow to 2000m 3 / h. Oxidation ends when the oxygen content of the tail gas reaches 18-20%.
[0024] 3. The oxide material is dehydr...
Embodiment 2
[0026] The preparation of embodiment 2 partial anhydrides
[0027] The main production process steps are as follows:
[0028] 1. Put 200Kg of mesitylene and 2000Kg of acetic acid into the mixing kettle from the metering tank, then add 4Kg of cobalt acetate and 4Kg of tetrabromoethane. Control the mixing temperature to 100°C, start stirring and mixing.
[0029] 2. Put the mixed material into the oxidation tower, control the oxidation temperature to 180°C, and the pressure to 0.4Mpa, introduce air to trigger a stage of oxidation reaction, control the oxidation temperature to 140-180°C, and the oxidation pressure to 0.4-1.0Mpa. After triggering for 60 minutes, drop into the prepared catalyst, including 6Kg of cobalt acetate, 6Kg of tetrabromoethane, and 10Kg of manganese acetate. Control the oxidation temperature at 180-300°C, the pressure at 1.0-3.0Mpa, and adjust the air flow to 1000m 3 / h. Oxidation ends when the oxygen content of the tail gas reaches 18-20%.
[0030] 3. ...
Embodiment 3
[0032] The preparation of embodiment 3 partial anhydrides
[0033] The main production process steps are as follows:
[0034]1. Put 400Kg of mesitylene and 2400Kg of acetic acid into the mixing tank from the metering tank, then add 2.7Kg of cobalt acetate and 2.7Kg of tetrabromoethane. The temperature of the mixing tank was controlled at 80°C, and the stirring was started.
[0035] 2. Put the mixed material into the oxidation tower, control the temperature at 150°C, the pressure at 0.8Mpa, and let in air. A period of oxidation reaction is initiated, the oxidation temperature is controlled at 140-180°C, and the oxidation pressure is 0.4-1.0Mpa. After triggering for 50 minutes, drop into the prepared catalyst, including 3.3Kg of cobalt acetate, 3.3Kg of tetrabromoethane, and 6Kg of manganese acetate. Control oxidation temperature 180-300℃, pressure 1.0-3.0Mpa, adjust air flow to 2000m 3 / h. Oxidation ends when the oxygen content of the tail gas reaches 18-20%.
[0036] 3. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com