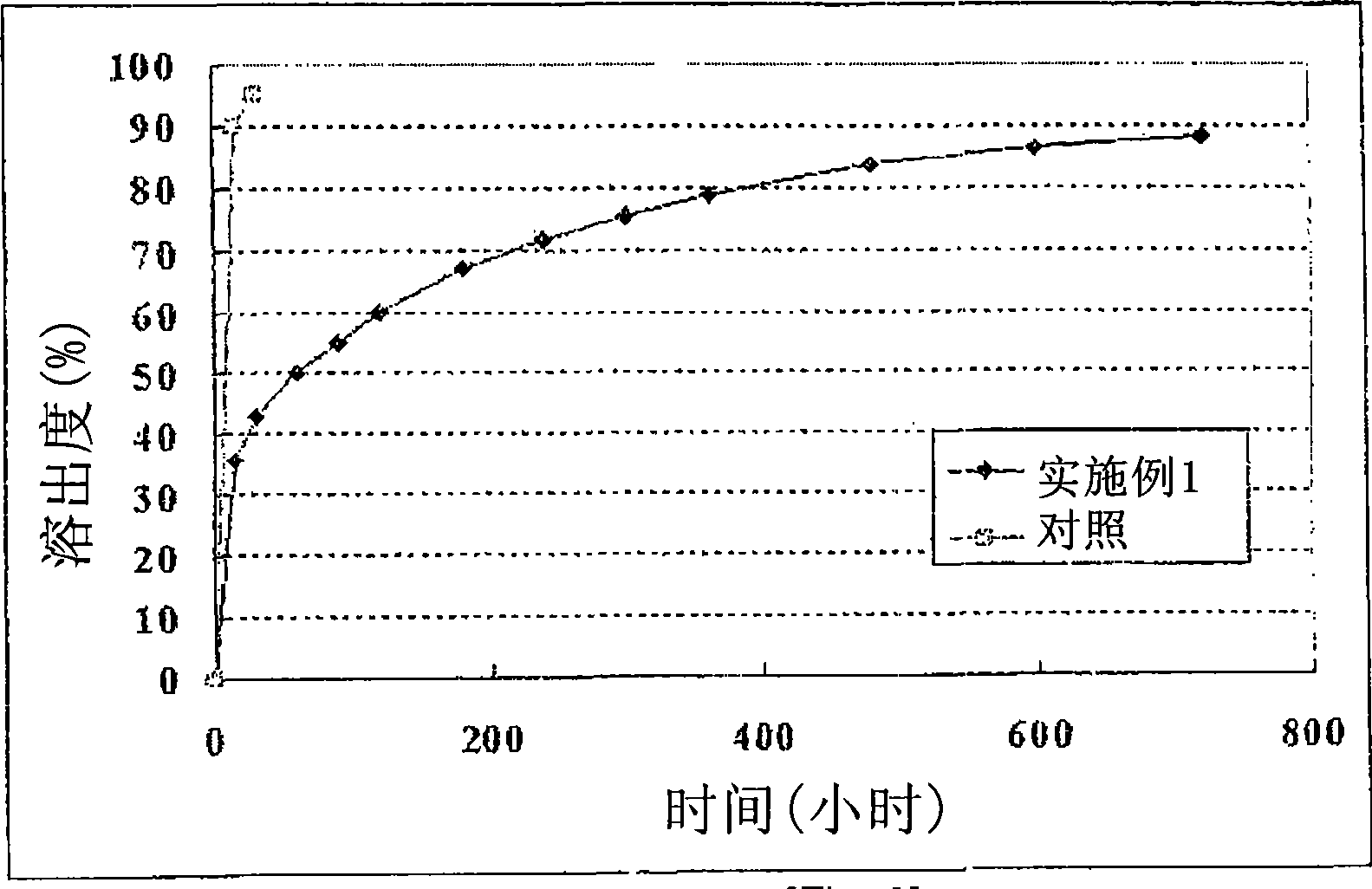
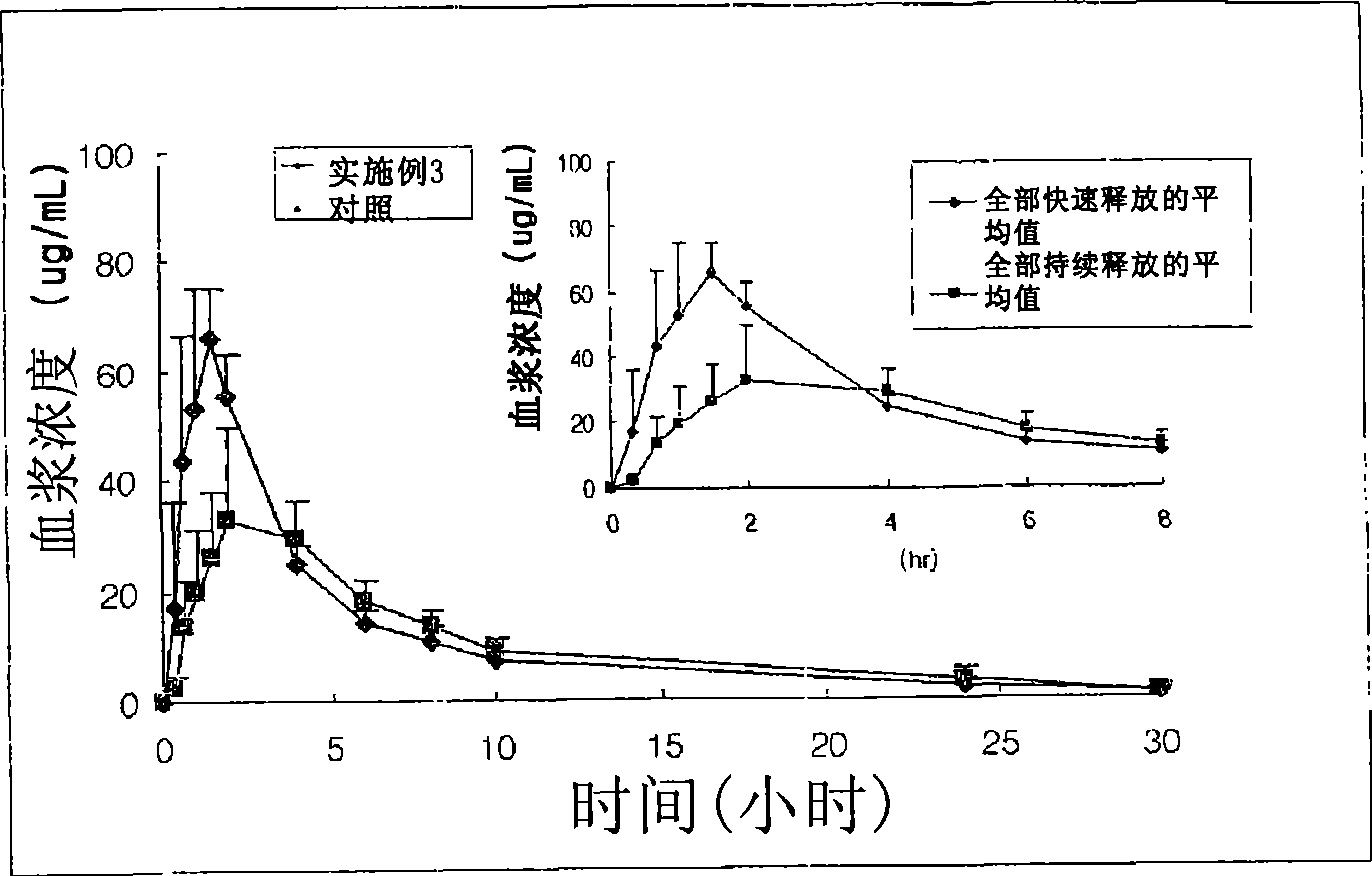
Multiple unit type sustained release oral formulation and process for the preparation thereof
A sustained-release, oral preparation technology, applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] In the preparation of granules, first, the water-insoluble polymer is dissolved in an organic solvent alone or dispersed in distilled water to prepare a solution or dispersion of the water-insoluble polymer, and then the Granules are prepared. As a method of producing granules, for example, a method of wet granulation or a method of dry granulation can be used. For the wet granulation method, a method using a fluidized bed granulator or a method using a high-speed mixer can be applied, while for the dry granulation method, another method using a belt granulation using a roller compactor is applied. , Direct tableting of water-insoluble polymer raw materials using excipients for direct tableting or similar methods. Specifically, in the case of using a fluidized bed granulator, the fluidized bed granulator is sufficiently dried and preheated under conditions of an inlet temperature in the range of 60 to 85°C and an outlet temperature of 30 to 65°C, and then Granules can...
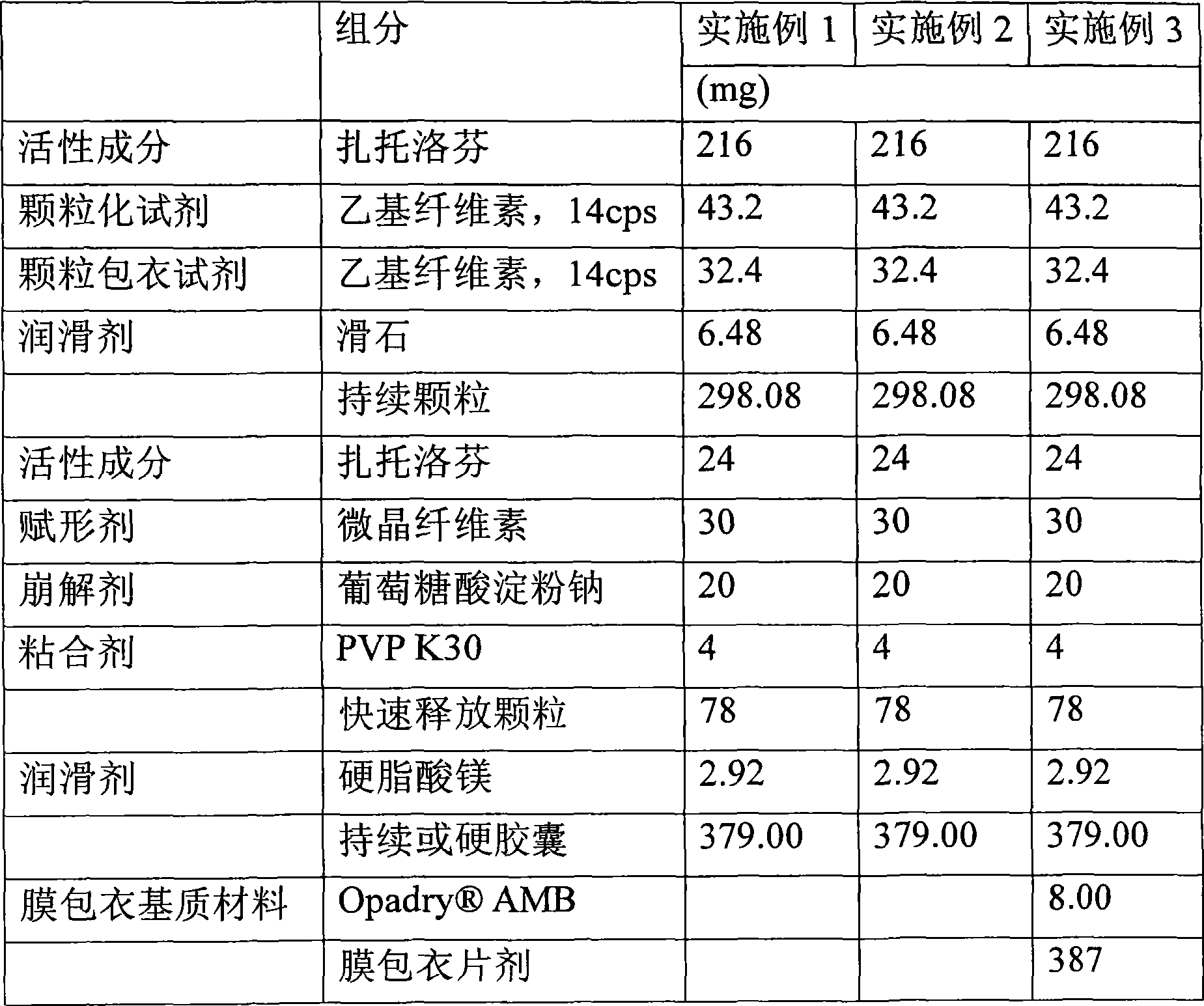
Embodiment 1
[0072] A. Preparation of Water-Insoluble Polymer Solutions
[0073] 100 g of ethyl cellulose with a viscosity of 14 cps, a water-insoluble polymer, was added to 1000 g of 80% aqueous ethanol, and then the mixture was stirred with a mechanical mixer at 1000 rpm for 30 to 60 minutes to prepare a water-insoluble polymer solution.
[0074] B. Preparation of Granules Containing Zaltoprofen and Water-Insoluble Polymer
[0075] The fluidized bed granulator was fully dried and preheated under conditions of an inlet temperature of 65°C and an outlet temperature of 30°C, followed by a solution of the resulting water-insoluble polymer ethylcellulose at an input rate of 720mL / hour Absorbed onto 500 g of zaltoprofen to produce 600 g of granules.
[0076] C. Preparation of Sustained Release Matrix Material Coating Solution
[0077] 75 g of ethyl cellulose having a viscosity of 14 cps, a sustained release matrix material, was added to 750 g of 80% aqueous ethanol, and then the mixture...
Embodiment 2
[0090] Embodiment 2: Preparation of multi-unit sustained-release capsules
[0091] The sustained-release pellets of zaltoprofen prepared in steps D and E of 298.08g embodiment 1, 78g quick-release granules and 2.92g magnesium stearate were jointly filled into No. 0 hard capsules of 379mg by free-falling method, To prepare sustained release capsules.
[0092]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com