Specific therapy using integrin ligands for treating cancer
An integrin and ligand technology, which can be used in peptide/protein components, medical preparations containing active ingredients, and pharmaceutical formulations, and can solve problems such as reducing efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
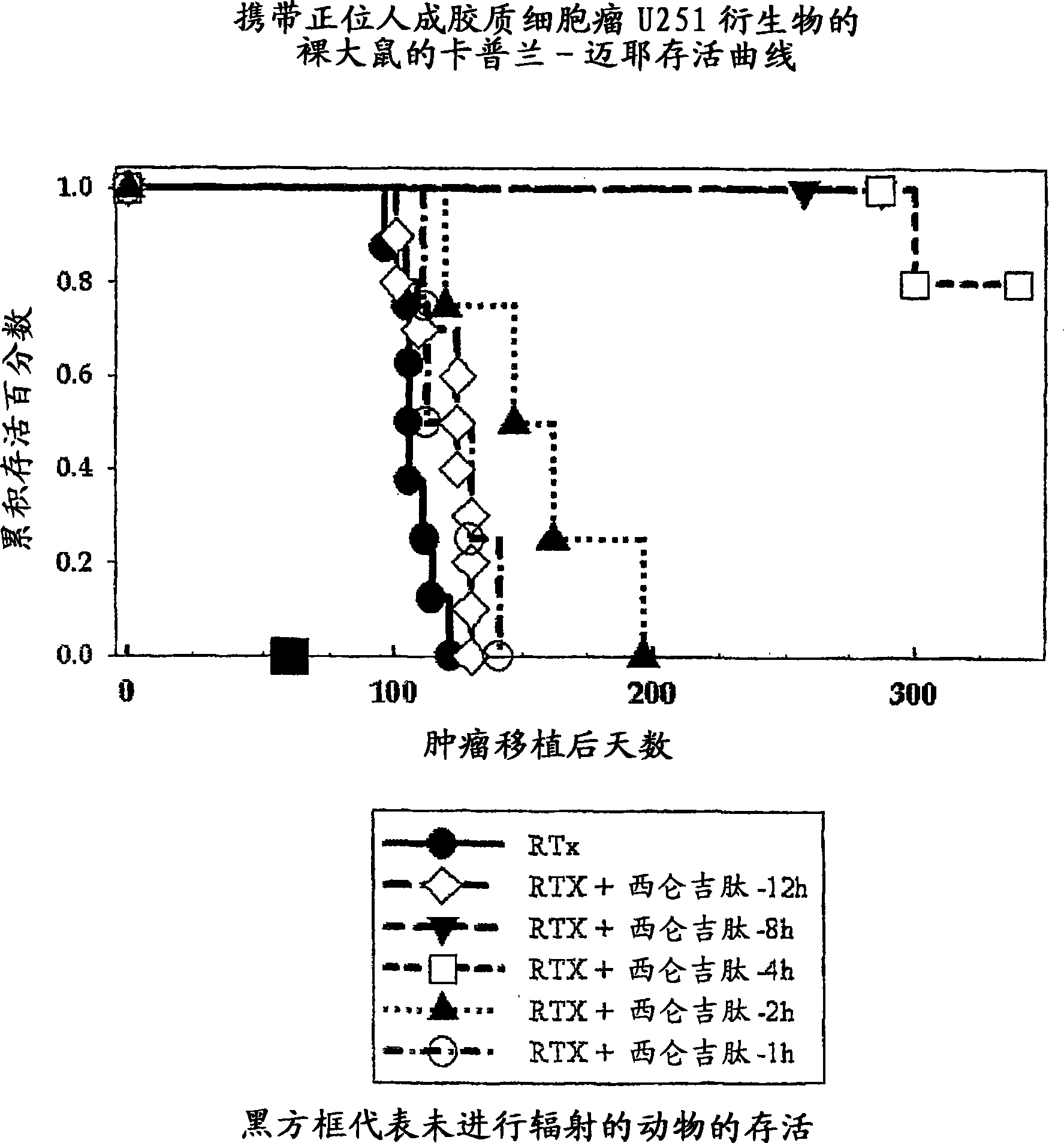
[0448]Example 1: Radiation Therapy, Cilengitide (= Cyclo-(Arg-Gly-Asp-DPhe-Nme-Val)) Sequence Experiment in a Rat Orthotopic Glioblastoma Model
[0449] NlH rnu nude rats were anesthetized, bound, and injected 5x10E5 U251 human glioblastoma cells suspended in 10ul of cell culture medium 1mm behind the orbit, 3mm and 2.5mm deep from the right side of the anterior blemish, wherein Injections were performed essentially as previously published (Engebraaten et al., 1999) using a #2701 Hamilton syringe fitted with a 26 gauge needle. After 14 days, before monotherapy with a single, collimated, dorsal-ventral 6MV x-ray beam so that 95-100% of the central axis dose of 25Gy hits the tumor volume (Kim et al., 1999) At time (8h, 4h, 2h, 1h), Cilengitide (4 mg / kg) in PBS was administered as an intraperitoneal bolus. Animals also received the same intraperitoneal bolus of cilengitide each day for the next 7 days. Animals were maintained under conditions of food and water ad libitum until ...
Embodiment 2
[0463] Example 2: Phase IIa trial of cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) mono-agent therapy in patients with recurrent glioblastoma
[0464] Background: This phase IIa study was designed to evaluate the cyclic RGD pentapeptide, cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val), inhibitor of integrins avβ3 and avβ5) as a single Safety, toxicity and clinical activity of the active agent at doses of 500 mg and 2000 mg in patients (pts) with relapsed glioblastoma (GBM).
[0465] METHODS: In this multicentre, open-label, randomized and uncontrolled study, pts with recurrent GBM and detectable disease following previous treatment with temozolomide and radiation therapy were randomized to receive a dose of 500 mg Or cilengitide 2000 mg intraperitoneally twice a week until disease progression. Independent blinded examinations were performed for histopathological diagnosis and MRI imaging. The primary endpoint was progression-free survival (PFS) at 6 months (mths). Second...
Embodiment 3
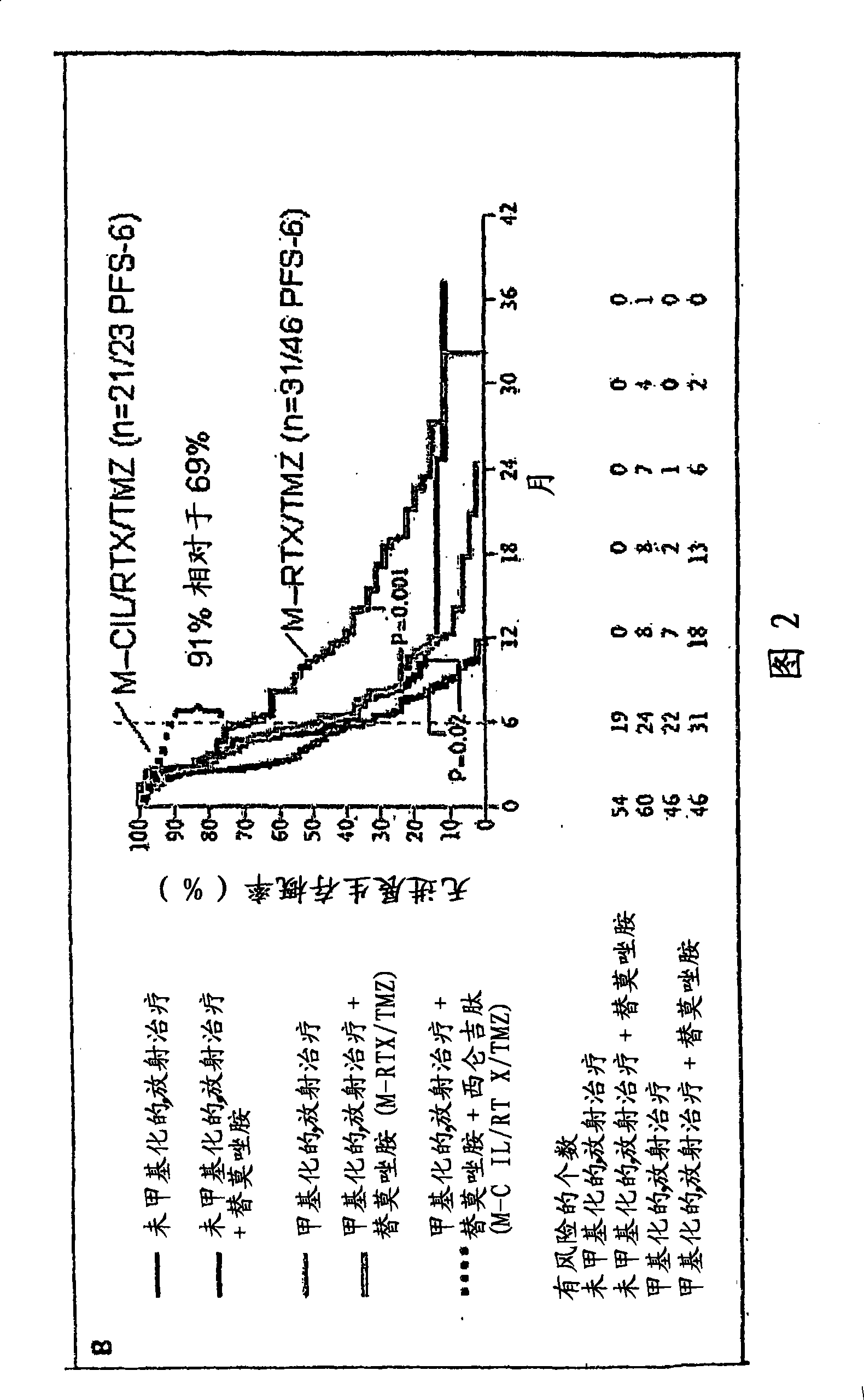
[0468] Example 3: Use of Cilengitide (=Cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) and Temozolomide and Simultaneously in Patients with Newly Diagnosed Glioblastoma (GBM) Radiation therapy followed by a phase I / IIa trial of maintenance temozolomide and cilengitide
[0469] Objective: To evaluate the combination of cyclic RGD pentapeptide-cilengitide (=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val), integrin, in addition to standard temozolomide (TMZ) and radiation therapy (RT). Inhibitors of proteins avβ3 and avβ5) safety, toxicity and efficacy.
[0470] Patients and methods: 52 patients (PS 0-1: 92%, 2: 8%; median age 57 years) after biopsy (n=9 / 17%) or tumor resection (n=43 / 83%) Standard TMZ / RT therapy (Stupp et al NEJM 2005). Additionally cilengitide (500 mg iv, twice / week) was started one week prior to TMZ / RT and was administered throughout the duration of chemotherapy or until progression. The primary endpoint was progression-free survival at 6 months (target: 65%). Patients were followed up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com