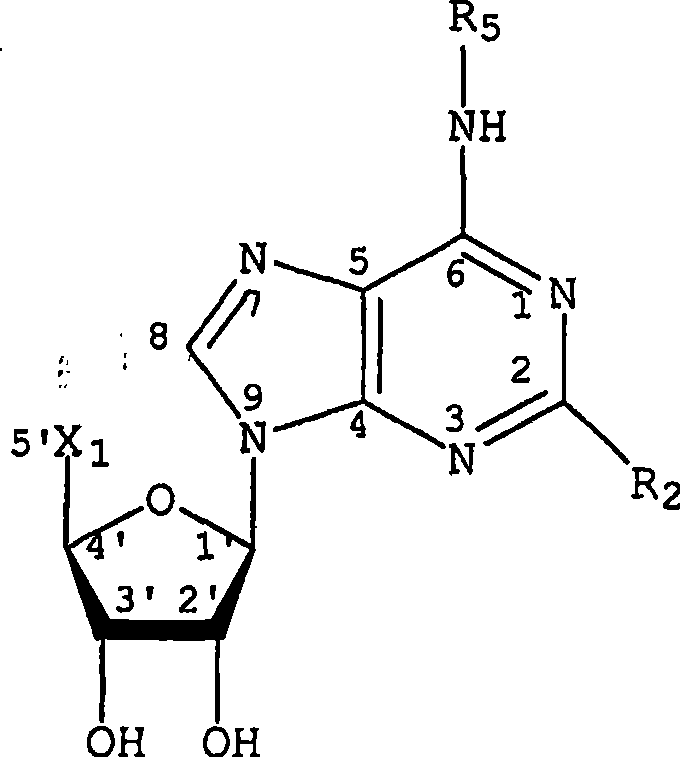
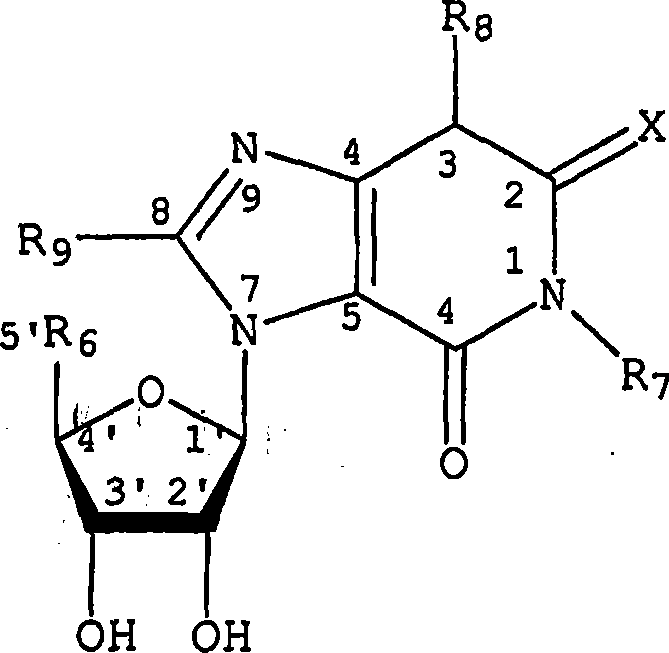
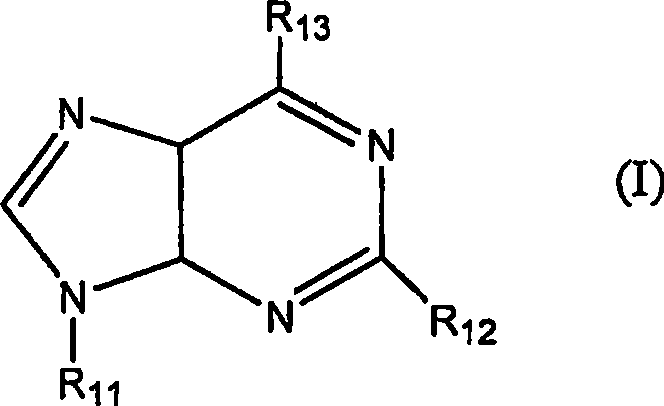
Adenosine a3 receptor agonists for the treatment of dry eye disorders
An adenosine receptor and agonist technology, applied in the field of compounds for the treatment of dry eye, can solve problems that cannot be prevented or reversed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0152] IB-MECA improves dry eye symptoms in rheumatoid arthritis patients
[0153] drug:
[0154] A used 3 AR agonists are clinical grade compounds traditionally known as 1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl -D-Ribofuranamide or N 6 -(3-iodophenyl)-adenosine-5′-N-methyluronic acid amide (IB-MECA), which was synthesized by Can-Fite BioPharma in accordance with Albany Molecular ResearchInc, Albany, NY, USA Good Clinical Practice (GMP).
[0155] IB-MECA is formulated as oval-shaped softgel capsules. The capsules contain a solution of IB-MECA in CremophorRH 40 and Miglyol 812. The capsules contain 0.1, 1 or 4 mg doses of IB-MECA, the exact composition of each capsule type is shown in Tables 1-3 below:
[0156] Table 1: Composition of 0.1 mg IB-MECA Softgel Capsules
[0157] Element capsule %W / W IB-MECA 0.105mg 0.021 Polyoxyl 45 castor oil,
USP (Cremophor RH 40) 326.495mg
65.299
Miglyol 812 173.400mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com