High-purity cefmenoxime hydrochloride and preparation thereof
A cefmenoxime hydrochloride, high-purity technology, applied in the field of medicine, can solve the problems of low purity, poor stability, and large side effects of cefmenoxime hydrochloride, and achieve the effects of improving clarity, stability, and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
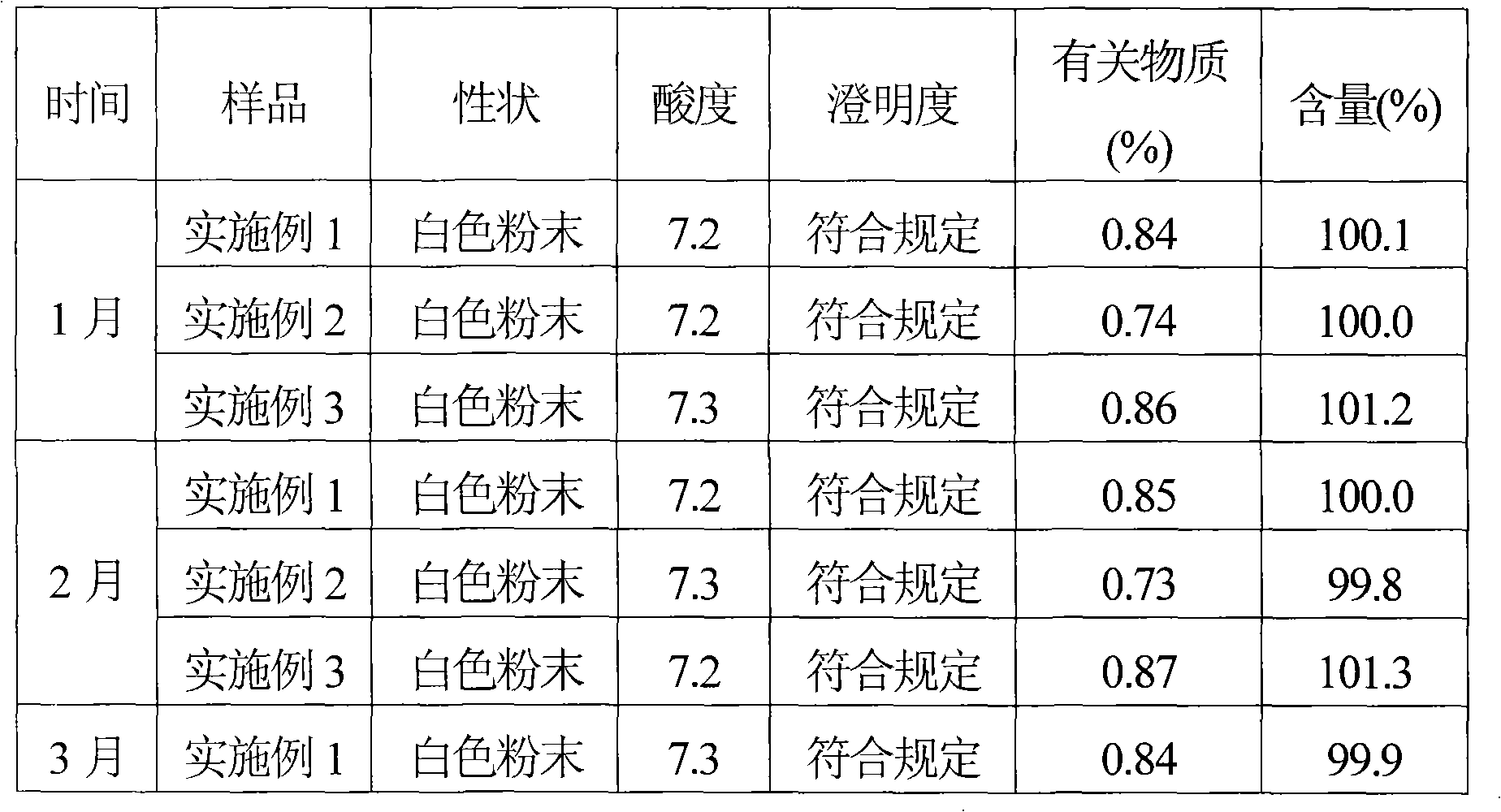
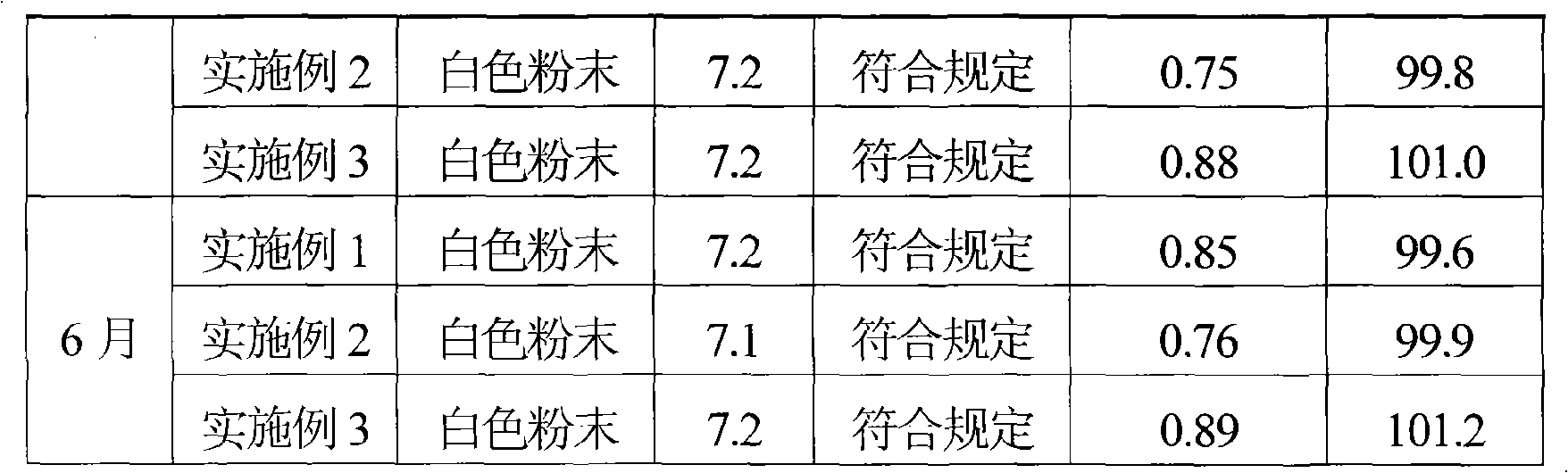
[0039] Take 500 g of cefmenoxime and dissolve it in 2000 ml of purified water, add 2 mol / L hydrochloric acid solution to adjust the pH of the solution to 2.8, then adsorb it with D1300 macroporous resin, and elute with distilled water until there is almost no cefmenoxime hydrochloride in the eluent. Collect the eluate, concentrate the eluate by evaporation under reduced pressure, and use SephadexC 15Carry out column chromatography on a cross-linked Sephadex column as a filler, elute with 0.1mol / L ammonium formate aqueous solution, and the elution temperature is 20°C, collect the part with an integral area ratio of 99% or more detected by HPLC, and add a solution volume of 0.2 % (g / ml) activated carbon, decolorized at room temperature for 30 minutes, sterilized by filtration with a 0.22 μm microporous membrane, and freeze-dried to obtain 441.2 g of cefmenoxime hydrochloride with a purity of 99.3% and a yield of 85.2%.
[0040] After the above-mentioned cefmenoxime hydrochloride...
Embodiment 2
[0042] Take 1000 g of cefmenoxime hydrochloride produced by Suzhou Zhonglian Chemical Pharmaceutical Co., Ltd., stir and dissolve it in 4000 ml of purified water, add 3 mol / L hydrochloric acid solution to adjust the pH of the solution to 3.1, adsorb with D1300 macroporous resin, and elute with distilled water until the There is almost no cefmenoxime hydrochloride in the dehydration. Collect the eluate, concentrate the eluate by evaporation under reduced pressure, and use SephadexC 25 Carry out column chromatography on the cross-linked Sephadex column as filler, elute with 0.2mol / L ammonium acetate aqueous solution, the elution temperature is 25°C, collect the part with an integral area ratio of 99% or more detected by HPLC, add 0.2% of the solution volume % (g / ml) activated carbon, decolorized at room temperature for 30 minutes, sterilized by filtration with a 0.22um microporous membrane, and freeze-dried to obtain 844.0 g of cefmenoxime hydrochloride with a purity of 99.5% an...
Embodiment 3
[0045] Take 800 g of cefmenoxime and dissolve it in 3000 ml of purified water, add 1 mol / L hydrochloric acid solution to adjust the pH of the solution to 2.5, then adsorb it with D1300 macroporous resin, and elute with distilled water until there is almost no cefmenoxime hydrochloride in the eluent. Collect the eluate, concentrate the eluate by evaporation under reduced pressure, and use SephadexC 50 Carry out column chromatography on the cross-linked Sephadex column as filler, elute with 0.2mol / L ammonium propionate aqueous solution, the elution temperature is 23°C, collect the part with an integral area ratio of 99% or more detected by HPLC, add the volume of the solution 0.2% (g / ml) activated carbon, decolorized at room temperature for 30 minutes, sterilized by filtration with a 0.22um microporous membrane, and freeze-dried to obtain 672.77g of cefmenoxime hydrochloride, with a purity of 99.2% and a yield of 81.2% .
[0046] After the above-mentioned cefmenoxime hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com