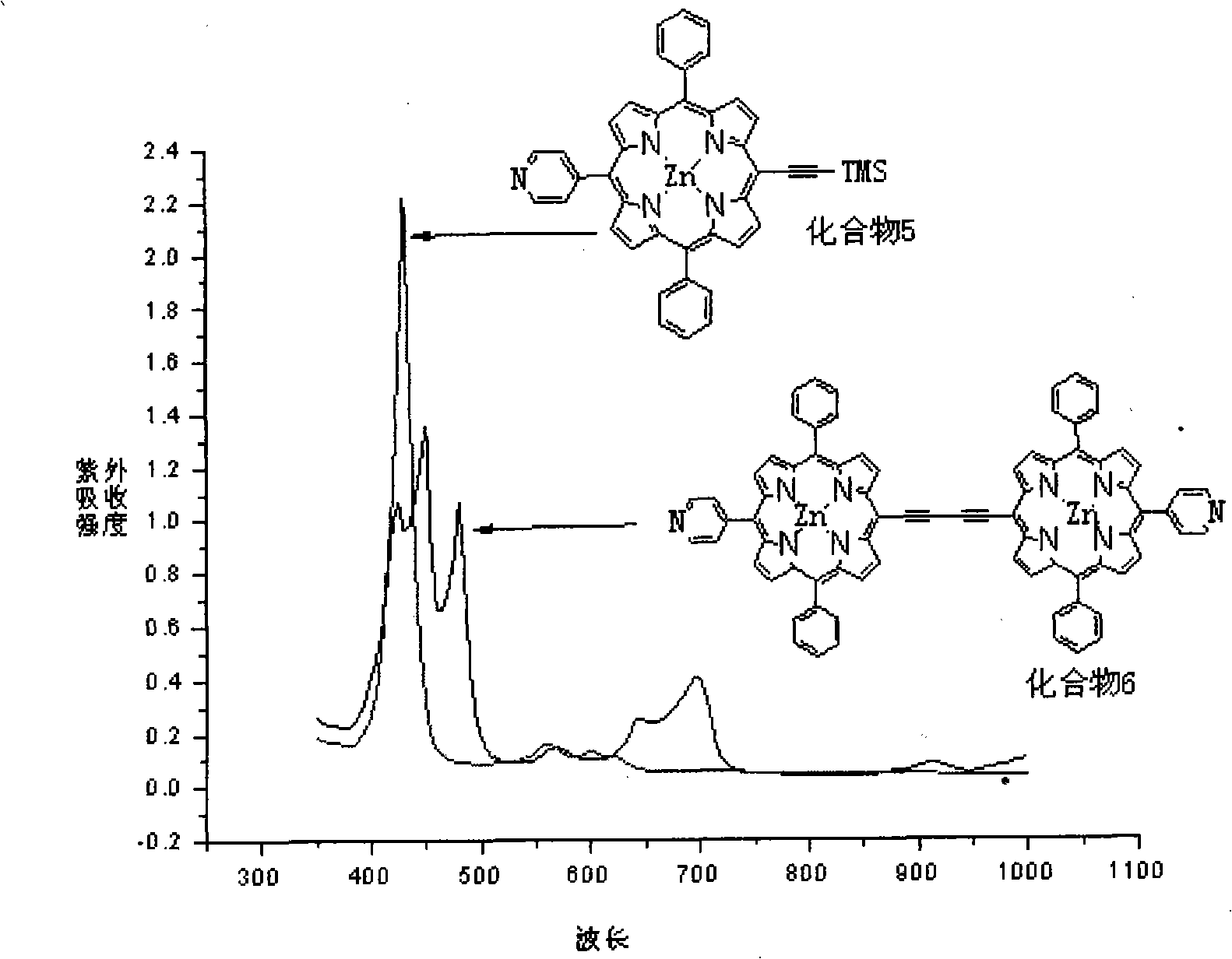
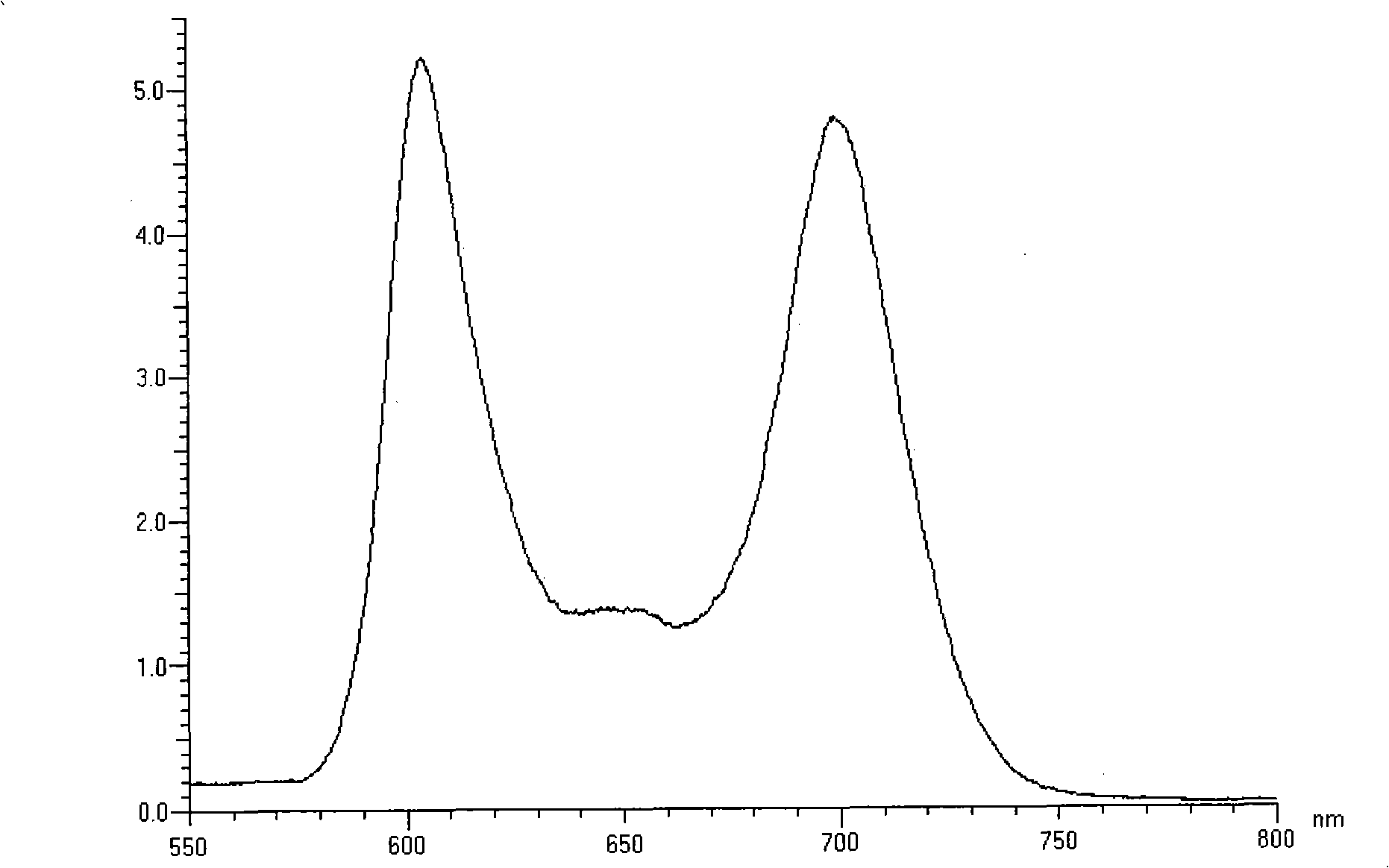
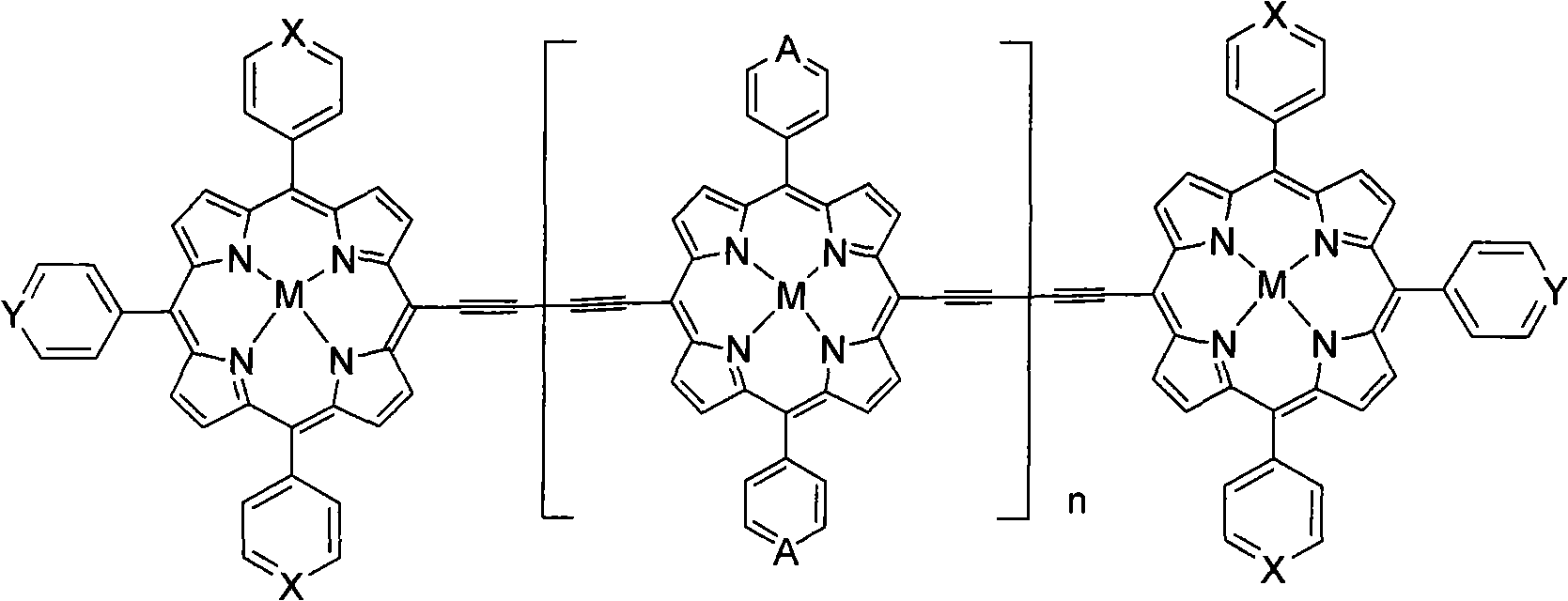
Near infrared fluorescent compounds of porphyrins connected with alkynyl and preparation method
A fluorescent compound, near-infrared technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of limiting the sensitivity of fluorescent probes, unsatisfactory photophysical properties, and reducing background interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] In the preparation process of the present invention, the input involved is only the ratio of the amount of one of the substances. In a specific reaction, we can enlarge or reduce the ratio of the amount of this substance.
[0046]
[0047] The preparation method of two{[5,5'-10,20-diphenyl-15-(4-pyridyl)porphyrin]Zn(II)]}butadiyne
Embodiment 1
[0050] Synthesis of 5-Phenyldipyrromethane (Compound 3)
[0051] Add (150 ml, 2.16 mol) freshly distilled pyrrole and (6.0 ml, 59 mmol) benzaldehyde in a 250 ml two-neck round bottom flask, protect with nitrogen for 15 minutes, add (0.45 ml, 5.8 mmol) three Fluoroacetic acid, continue to react for 15 minutes, and distill off excess pyrrole under reduced pressure to obtain dark yellow oil, which is dissolved in dichloromethane, purified by silica gel column chromatography, and eluted with dichloromethane to obtain 5-phenyldipyrrole 8.6 grams of methane, yield 65%. 1 HNMR (300MHz, CDCl 3)δ: 5.44 (s, 1H), 5.83 (br, s, 2H), 6.15 (d, 2H), 6.69 (br, s, 2H), 7.20-7.34 (m, 5H), 7.90 (br s, 2H ); ESI-MS (CHCl 3 ): 223.3 ([M+H] + ).
Embodiment 2
[0053] Synthesis of 5-Phenyldipyrromethane (Compound 3)
[0054] Add (150 ml, 2.16 mol) freshly distilled pyrrole and (6.0 ml, 59 mmol) benzaldehyde in a 250 ml two-necked round bottom flask, react at 20°C, protect with nitrogen for 40 minutes, add (0.45 ml , 5.8 mmol) trifluoroacetic acid, continued to react for 10 minutes, and removed excess pyrrole by distillation under reduced pressure to obtain dark yellow oil, which was dissolved in dichloromethane, purified by silica gel column chromatography, and eluted with dichloromethane to obtain 5-Phenyldipyrromethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com