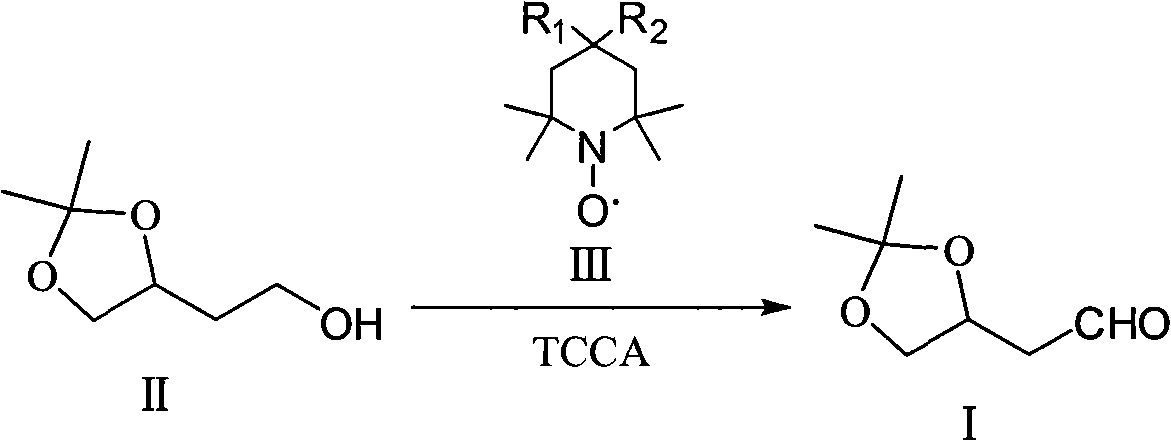
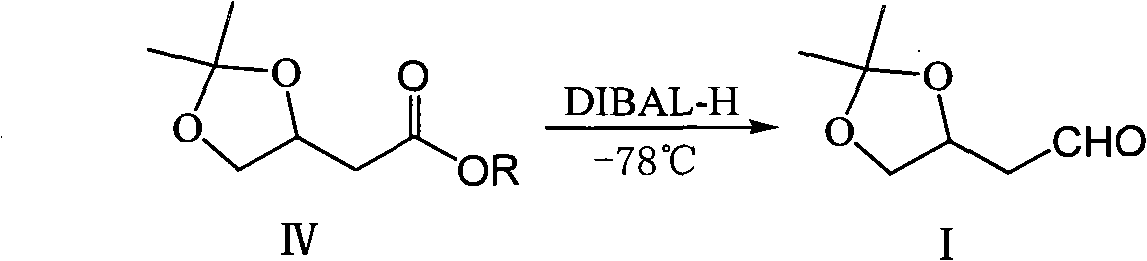
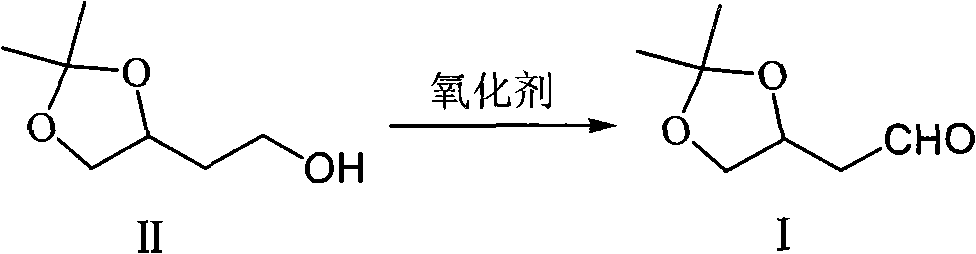
Method for preparing 3,4,-O-isopropylidene-3,4-dihydroxy butyraldehyde
A technology of isopropylidene and hydroxybutyraldehyde, applied in the direction of organic chemistry, can solve the problems of low yield and inability to oxidize raw materials, and achieve the effect of small dosage, low price and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 120ml of dichloromethane and 10.0g of (S)-1,2-O-isopropylidene-1,2,4-butane in sequence to a 250ml four-necked bottle equipped with electromagnetic stirring, a thermometer and a calcium chloride drying tube Triol (II), 11.23g sodium acetate and 11mg TEMPO were cooled in an ice-water bath to an internal temperature of 0-5°C, and then 7.07g TCCA (trichloroisocyanuric acid, content: 90%, the same below) was added, and the ice-water bath was removed. After reacting at room temperature for 15 minutes, the reaction solution was filtered, and the obtained filtrate was subjected to saturated Na 2 CO 3 Aqueous solution, saturated brine washing, anhydrous MgSO 4 Dry and distill under reduced pressure to obtain 7.73g colorless transparent liquid product (S)-3,4-O-isopropylidene-3,4-dihydroxybutyraldehyde (I) [GC: 98.0%; bp: 56- 57℃ / 3mmHg; 1 H-NMR (CDCl 3, 400MHZ): 1.36(3H, s), 1.41(3H, s), 2.60-2.85(2H, m), 3.58(1H, dd), 4.17(1H, dd), 4.52(1H, m), 9.79( 1H, s); ESI-MS m / z...
Embodiment 2
[0035] Add 120ml methylene chloride, 8.42g sodium acetate, 10.0g (S)-1,2-O-isopropylidene-1 successively in a 250ml four-necked bottle equipped with electromagnetic stirring, a thermometer and a calcium chloride drying tube, 2,4-butanetriol (II) and 10.61g TCCA, cool in an ice-water bath to an internal temperature of 0-5°C, add dropwise a solution of 0.11g TEMPO in 5ml of dichloromethane, remove the ice-water bath, react at room temperature for 15 minutes, and then filter The reaction solution, the resulting filtrate was successively subjected to saturated Na 2 CO 3 Aqueous solution, saturated brine washing, anhydrous MgSO 4 After drying, the solvent was distilled off under reduced pressure to obtain 8.71 g of a colorless and transparent liquid product (S)-3,4-O-isopropylidene-3,4-dihydroxybutyraldehyde (I).
Embodiment 3
[0037] Add 120ml of dichloromethane and 10.0g of (R)-1,2-O-isopropylidene-1,2,4-butane in sequence to a 250ml four-necked bottle equipped with electromagnetic stirring, a thermometer and a calcium chloride drying tube Triol (II), 11.23g sodium acetate and 7.07g TCCA, cool in an ice-water bath to an internal temperature of 0-5°C, add 40mg TEMPO, remove the ice-water bath, react at room temperature for 10 minutes, and filter the reaction solution, and the obtained filtrate is saturated in turn Na 2 CO 3 Aqueous solution, saturated brine washing, anhydrous MgSO 4 Drying, solvent removal under reduced pressure obtains 8.56g colorless transparent liquid product (R)-3,4-O-isopropylidene-3,4-dihydroxybutyraldehyde (I) [ 1 H-NMR (CDCl 3 , 400MHZ): 1.35(3H, s), 1.41(3H, s), 2.57-2.85(2H, dd), 3.58(1H, m), 4.17(1H, m), 4.51(1H, m), 9.77( 1H,t)].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com