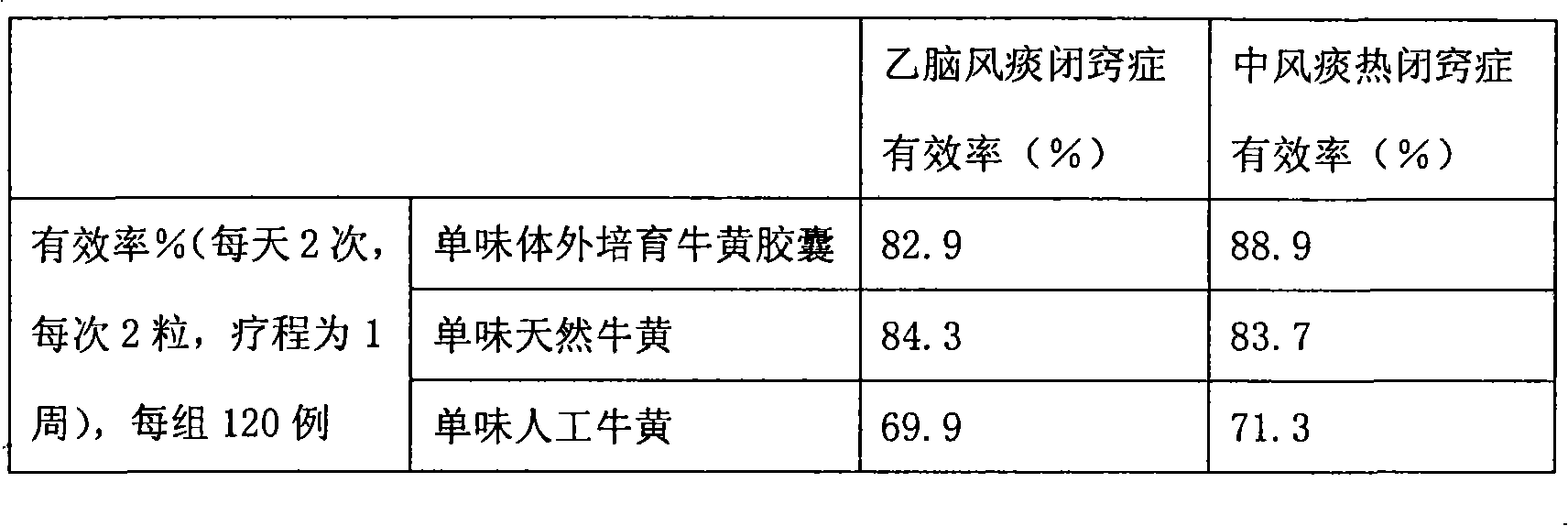
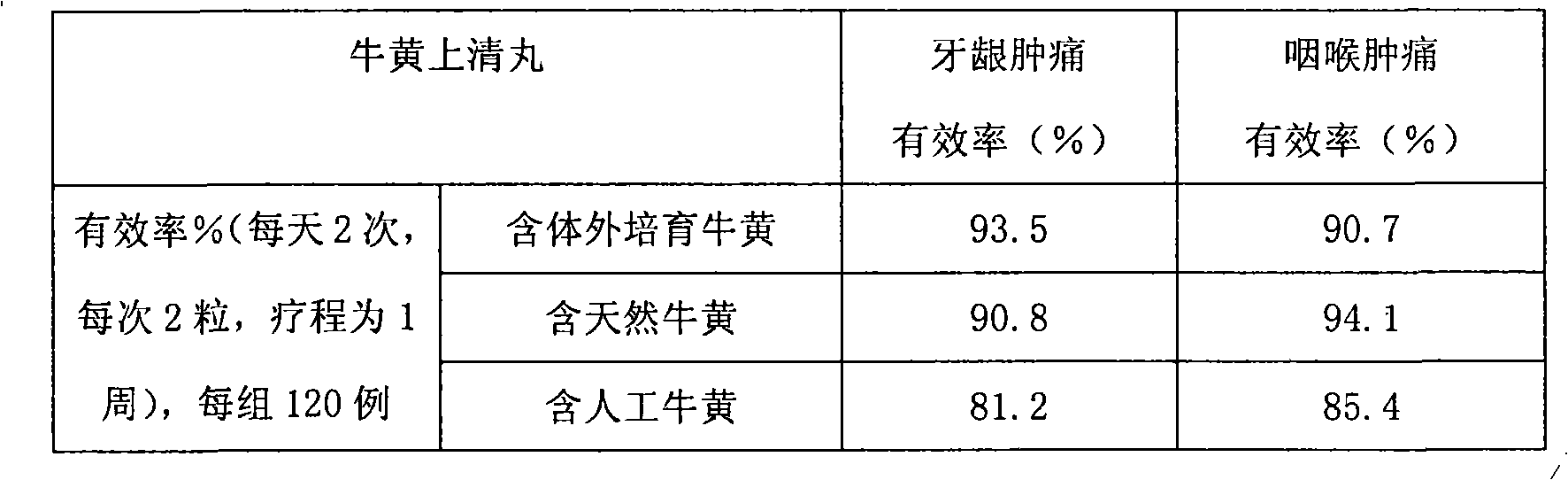
Bezoar vitro bred and solid oral preparation containing the bezoar
A technology for cultivating bezoar and solid preparations in vitro, which is applied in the directions of medical preparations containing active ingredients, anti-inflammatory agents, and pill delivery, etc., to achieve the effects of easy control and excellent clinical efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Take 1000ml of fresh ox bile.
[0047] (2) Add the following components:
[0048] Bilirubin 300g
[0049] Cholic acid 100g
[0050] Deoxycholic acid 50g
[0051] Cholesterol 10
[0054] Calcium hydrogen phosphate 10g
[0055] Taurine 15g
[0056](3) Mix the above components with 100ml of β-glucuronidase suspension evenly, seal it, keep it in a shaking water bath at 37±1°C for 5 hours, then measure the pH value of ox bile every 1 hour, when the pH is at 3 ~4:00, stop shaking the water bath.
[0057] (4) The above-mentioned mixture is separated with a centrifuge to remove the precipitate, washed with distilled water, and the content of bilirubin and cholic acid is measured, and bilirubin and cholic acid are added to make the bilirubin content 41% and the content of cholic acid in the mixture 12.6%.
[0058] (5) Add an appropriate amount of distilled water to the mixture obtained in step (4) (accordin...
Embodiment 2
[0062] (1) Take 1000ml of fresh ox bile.
[0063] (2) Add the following components:
[0064] Bilirubin 310g
[0065] Cholic acid 80g
[0066] Deoxycholic acid 40g
[0067] Cholesterol 15
[0070] Calcium hydrogen phosphate 10g
[0071] Taurine 25g
[0072] (3) Mix the above components with 100ml of β-glucuronidase suspension evenly, seal it, keep it in a shaking water bath at 37±1°C for 5 hours, then measure the pH value of ox bile every 1 hour, when the pH is at 3 ~4:00, stop shaking the water bath.
[0073] (4) above-mentioned mixture is separated precipitation with centrifuge, washes with distilled water, measures bilirubin and cholic acid content, adds bilirubin and cholic acid, makes bilirubin content be 43% in the mixture, the content of cholic acid The content is 11.3%.
[0074] (5) Add an appropriate amount of distilled water to the mixture obtained in step (4) (according to the ratio of adding 50ml o...
Embodiment 3
[0078] Acetaminophen 250g
[0079] Chlorpheniramine Maleate 1g
[0080] Caffeine 15g
[0081] In vitro cultured bezoar 10g
[0082] Ethanol (40%) appropriate amount
[0083] Magnesium stearate 0.4g
[0084]
[0085] Makes 1000 capsules
[0086] Add the weighed chlorpheniramine maleate, caffeine, and in vitro cultured bezoar (prepared according to the method in Example 1) into a high-efficiency wet mixer and mix for 10 minutes, then mix with acetaminophen in equal increments uniform. Add the prepared ethanol with a weight percentage of 40%, adjust the rotating speed of the high-efficiency wet mixing granulator, make granules, dry and granulate. After weighing the prescribed amount of magnesium stearate and mixing the granules evenly, fill the No. 1 capsule to get final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com