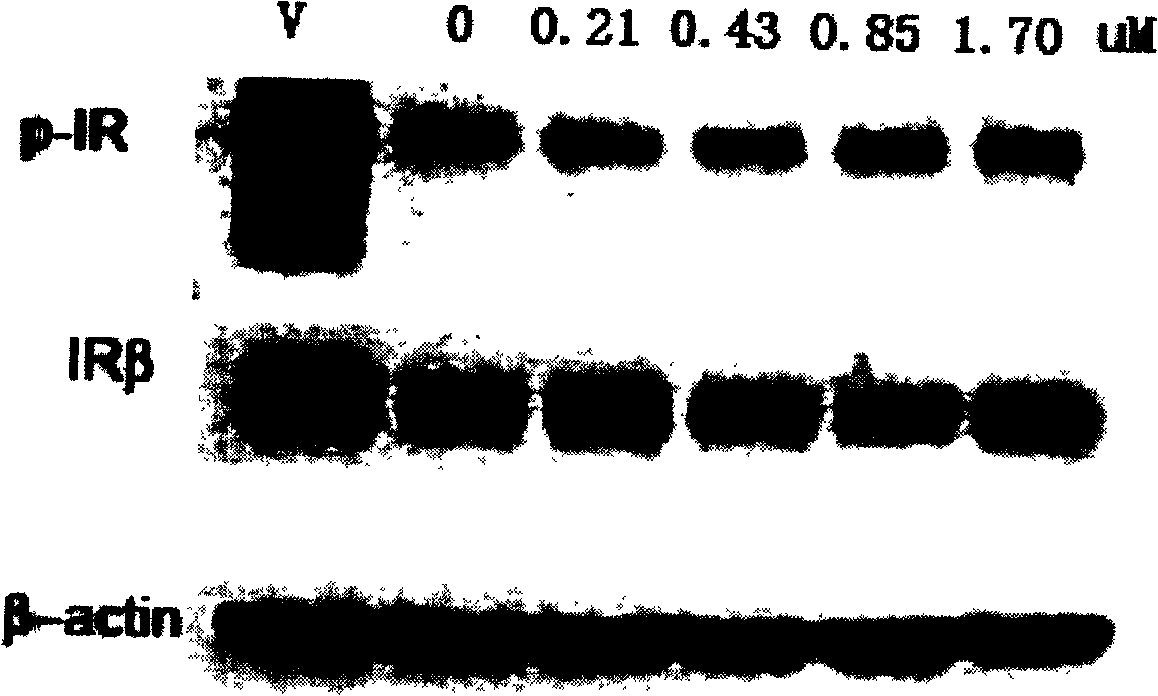
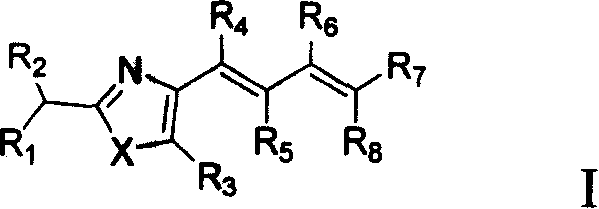
Protein-tyrosine-phosphatase 1B inhibitor and its preparation method and use
A C1-C6, halogen atom technology, applied in the delay or treatment of diseases mediated by PTP1B, type II diabetes and obesity drugs, insulin sensitizers, can solve difficult drug candidate compounds and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097]
[0098] step one:
[0099] Suspend 55mmol tyrosine in 100mL water and 100mL 1,4-dioxane, add 110mmol triethylamine, react for 1h, then add 83mmol (Boc) 2 O, react for 20h, spin dry. Dilute with ice water, extract and wash with ethyl acetate, adjust the pH of the aqueous phase to 2, and extract with ethyl acetate. The concentrated product was dissolved in 100 mL DME, 0.22 mol imidazole was added, and after stirring for 10 minutes, 0.1 mol TBSCl was added and reacted overnight. Quench with ice water, extract with ether, and concentrate to obtain compound 14 (yield 80%).
[0100] Dissolve 32 mmol of compound 14 in 200 mL of redistilled DME, add 32 mmol of N-methylmorpholine and 32 mmol of isobutyl chloroformate at -13°C, react at room temperature for 1 h, bubbling in ammonia gas for 4 h, quench with ice water, and extract with chloroform. The concentrated product was dissolved in 200 mL of redistilled DME, added 16 mmol Lawesson reagent, reacted for 5 hours, quenched with i...
Embodiment 2~13
[0114] The final products of Examples 2-13 in Table 1-1 were prepared by a method similar to Example 1.
[0115] Table 1-1 Final product (R 5 =R 7 =Me)
[0116]
[0117]
[0118]
Embodiment 14
[0120]
[0121] The preparation method of compound 21 is the same as in Example 1.
[0122] Dissolve 0.05mmol of compound 21 in 1mL of dichloromethane, add 0.3mL of trifluoroacetic acid dropwise, react for 2h, spin off the solvent and trifluoroacetic acid. Add 0.06mmol F 3 CCOOH, 0.12mmol EDCI, 0.01mmol DMAP and 2mL DMF were reacted overnight, quenched with ice water, extracted with ethyl acetate, concentrated, and passed through the column with petroleum ether: ethyl acetate=4:1 to obtain compound 23 (yield 50-90% ).
[0123] Compound 23 (WY558f)
[0124] 1 H NMR(CDCl 3 , 300MHz) data: δ 1.26 (t, 3H), 2.04 (s, 3H), 2.17 (s, 3H), 3.26 (dq, 2H), 4.21 (q, 2H), 5.02 (s, 2H), 5.47 (q, 1H), 6.27(s, 1H), 6.48(s, 1H), 6.88(d, 2H), 6.98(d, 2H), 7.04(s, 1H), 7.32-7.44(5H), 7.48( d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com